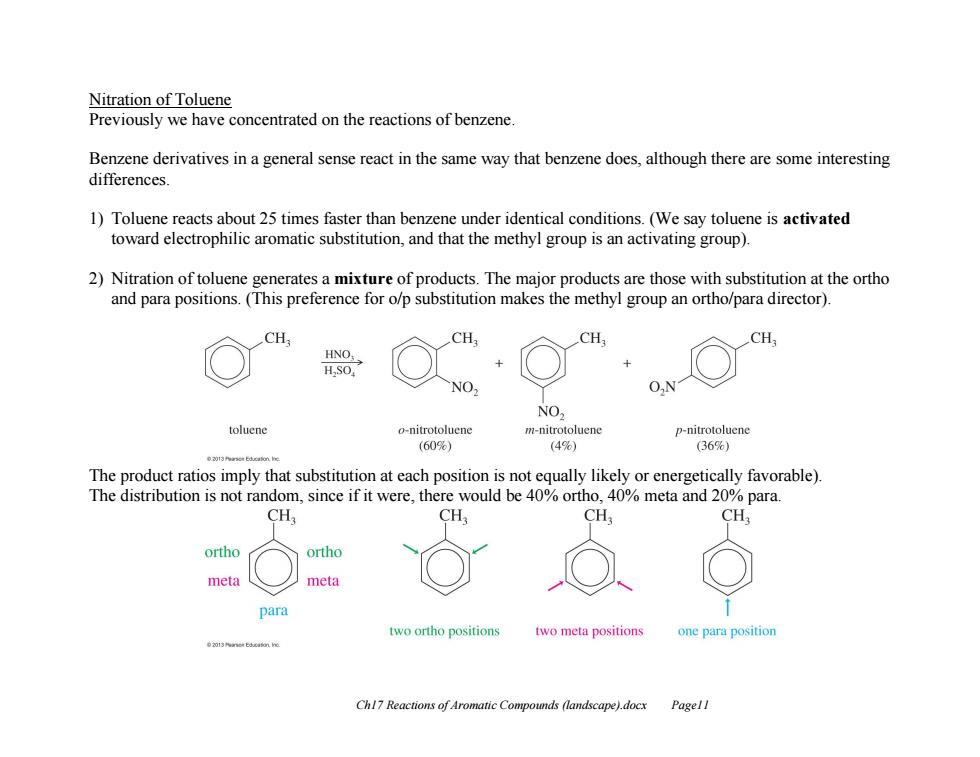
Nitration of Toluene Previously we have concentrated on the reactions of benzene. Benzene derivatives in a general sense react in the same way that benzene does,although there are some interesting differences 1)Toluene reacts about 25 times faster than benzene under identical conditions.(We say toluene is activated toward electrophilic aromatic substitution,and that the methyl group is an activating group). 2)Nitration of toluene generates a mixture of products.The major products are those with substitution at the ortho and para positions.(This preference for o/p substitution makes the methyl group an ortho/para director). CH CH. CH CH; HNO. H.SO. NO. NO, toluene o-nitrotoluene m-nitrotoluene p-nitrotoluene (600) (49%) (36%) The product ratios imply that substitution at each position is not equally likely or energetically favorable). The distribution is not random,since if it were,there would be 40%ortho,40%meta and 20%para. CH CH, CH, CH ortho ortho meta meta para two ortho positions two meta positions one para position Chl7 Reactions of Aromatic Compounds (landscape).docx Pagel!
Ch17 Reactions of Aromatic Compounds (landscape).docx Page11 Nitration of Toluene Previously we have concentrated on the reactions of benzene. Benzene derivatives in a general sense react in the same way that benzene does, although there are some interesting differences. 1) Toluene reacts about 25 times faster than benzene under identical conditions. (We say toluene is activated toward electrophilic aromatic substitution, and that the methyl group is an activating group). 2) Nitration of toluene generates a mixture of products. The major products are those with substitution at the ortho and para positions. (This preference for o/p substitution makes the methyl group an ortho/para director). The product ratios imply that substitution at each position is not equally likely or energetically favorable). The distribution is not random, since if it were, there would be 40% ortho, 40% meta and 20% para

We have already seen that the RDS for EAS is the first step,which requires the loss of aromaticity to generate the sigma complex. This step is also when the electrophile binds to the ring(i.e.governs the location of substitution) The enhanced rate and substitution pattern for toluene can be explained by considering the structures of the intermediate sigma complexes for substitution at each of the different positions. The RDS is highly endothermic,therefore according to Hammond's postulate(Ch4),the energy of the TS should resemble the energy of the product(in this case the product is actually an intermediate,the sigma complex). Thus it is reasonable to discuss the energies of the TS in terms of the stabilities of the sigma complexes(i.e.cation stabilities). When benzene reacts with the nitronium ion,the resulting sigma complex has the positive charge equally distributed over three secondary carbon atoms. Chl7 Reactions of Aromatic Compounds (landscape).docx Pagel2
Ch17 Reactions of Aromatic Compounds (landscape).docx Page12 We have already seen that the RDS for EAS is the first step, which requires the loss of aromaticity to generate the sigma complex. This step is also when the electrophile binds to the ring (i.e. governs the location of substitution). The enhanced rate and substitution pattern for toluene can be explained by considering the structures of the intermediate sigma complexes for substitution at each of the different positions. The RDS is highly endothermic, therefore according to Hammond's postulate (Ch 4), the energy of the TS should resemble the energy of the product (in this case the product is actually an intermediate, the sigma complex). Thus it is reasonable to discuss the energies of the TS in terms of the stabilities of the sigma complexes (i.e. cation stabilities). When benzene reacts with the nitronium ion, the resulting sigma complex has the positive charge equally distributed over three secondary carbon atoms
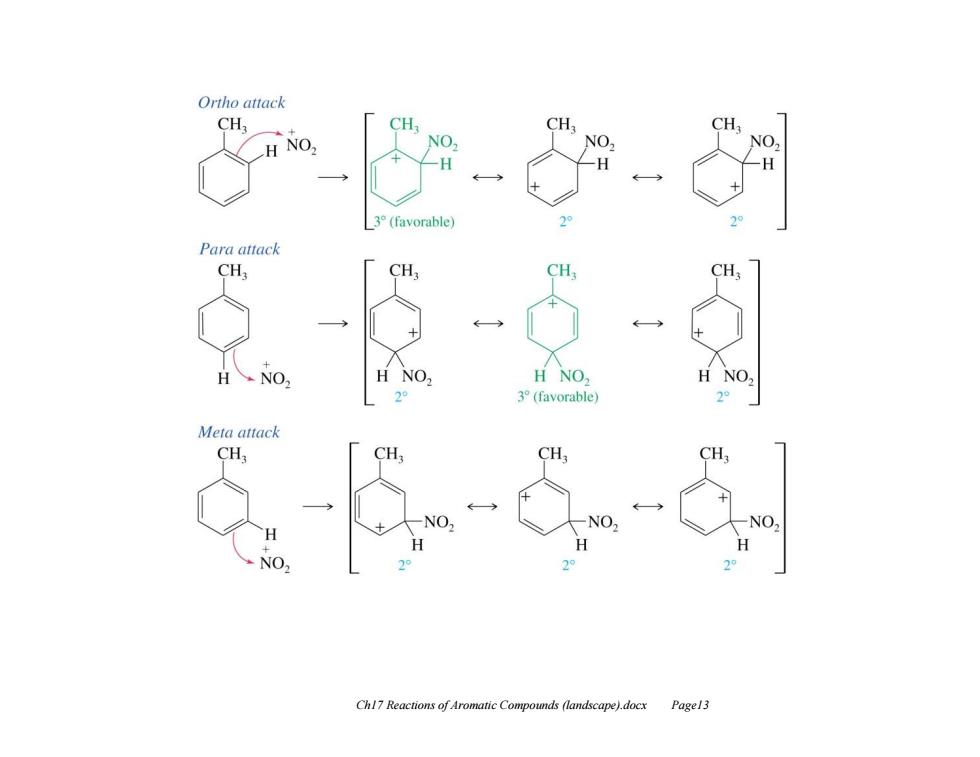
Ortho attack CH, CH CH3 CH3 H NO, NO H -H H 3°(favorable) 2 2 Para attack CH3 CH3 CH3 CH3 H NO, H NO, H NO 2° 3(favorable) 2° Meta attack CH3 CH CH CH NO. NO. Chl7 Reactions of Aromatic Compounds (landscape).docx Pagel3
Ch17 Reactions of Aromatic Compounds (landscape).docx Page13
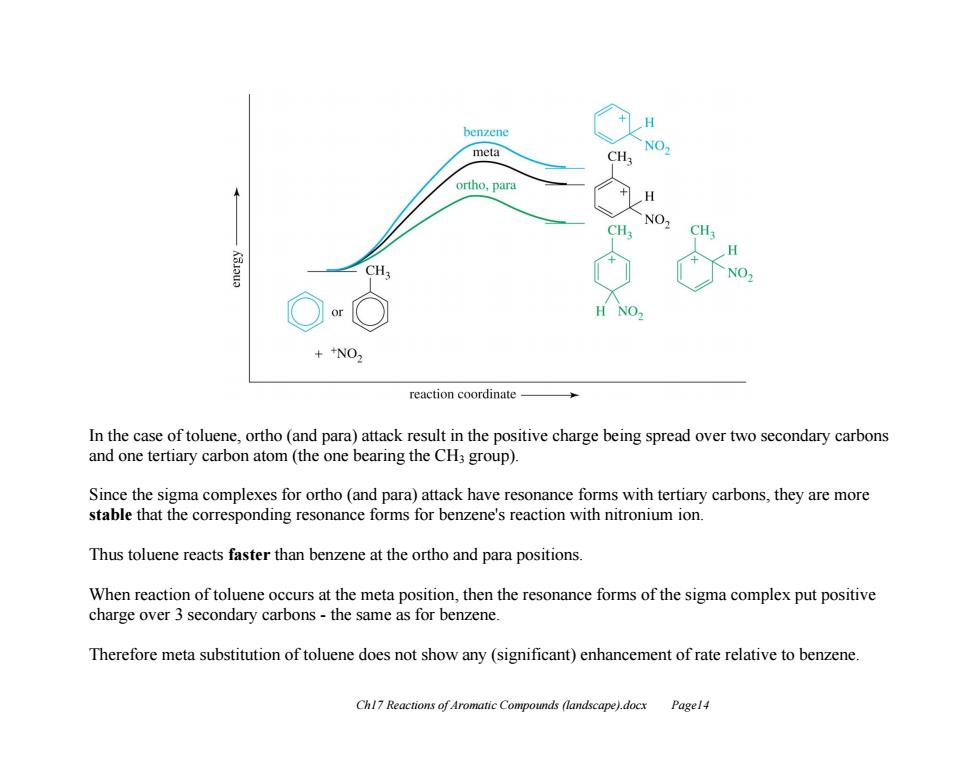
benzene meta NO ortho,para H NO CH, CH NO. ++NO2 reaction coordinate In the case of toluene,ortho(and para)attack result in the positive charge being spread over two secondary carbons and one tertiary carbon atom (the one bearing the CH3 group). Since the sigma complexes for ortho (and para)attack have resonance forms with tertiary carbons,they are more stable that the corresponding resonance forms for benzene's reaction with nitronium ion. Thus toluene reacts faster than benzene at the ortho and para positions. When reaction of toluene occurs at the meta position,then the resonance forms of the sigma complex put positive charge over 3 secondary carbons-the same as for benzene. Therefore meta substitution of toluene does not show any(significant)enhancement of rate relative to benzene. Chl7 Reactions of Aromatic Compounds (landscape).docx Pagel4
Ch17 Reactions of Aromatic Compounds (landscape).docx Page14 In the case of toluene, ortho (and para) attack result in the positive charge being spread over two secondary carbons and one tertiary carbon atom (the one bearing the CH3 group). Since the sigma complexes for ortho (and para) attack have resonance forms with tertiary carbons, they are more stable that the corresponding resonance forms for benzene's reaction with nitronium ion. Thus toluene reacts faster than benzene at the ortho and para positions. When reaction of toluene occurs at the meta position, then the resonance forms of the sigma complex put positive charge over 3 secondary carbons - the same as for benzene. Therefore meta substitution of toluene does not show any (significant) enhancement of rate relative to benzene

The methyl group is electron donating,and so stabilizes the intermediate sigma complex,and therefore the TS leading to it. This effect is pronounced in ortho and para attack since these give rise to resonance structures which contain tertiary carbons,and are therefore more stable. Meta substitution does not show these huge stabilizations,and is only slightly more stable then the unsubstituted benzene case Activating Ortho/Para Directing Substituents The results found with toluene are general for any alkyl substituted benzene undergoing EAS. Any alkyl benzene will under EAS faster than benzene itself,and will generate products that are primarily ortho and para. The alkyl group is an activating group,and is ortho and para directing. This is called inductive stabilization,since the alkyl group donates electron density through the o bond which attaches it to the benzene ring. Ch17 Reactions of Aromatic Compounds (landscape).docx Pagel5
Ch17 Reactions of Aromatic Compounds (landscape).docx Page15 The methyl group is electron donating, and so stabilizes the intermediate sigma complex, and therefore the TS leading to it. This effect is pronounced in ortho and para attack since these give rise to resonance structures which contain tertiary carbons, and are therefore more stable. Meta substitution does not show these huge stabilizations, and is only slightly more stable then the unsubstituted benzene case. Activating Ortho/Para Directing Substituents The results found with toluene are general for any alkyl substituted benzene undergoing EAS. Any alkyl benzene will under EAS faster than benzene itself, and will generate products that are primarily ortho and para. The alkyl group is an activating group, and is ortho and para directing. This is called inductive stabilization, since the alkyl group donates electron density through the bond which attaches it to the benzene ring