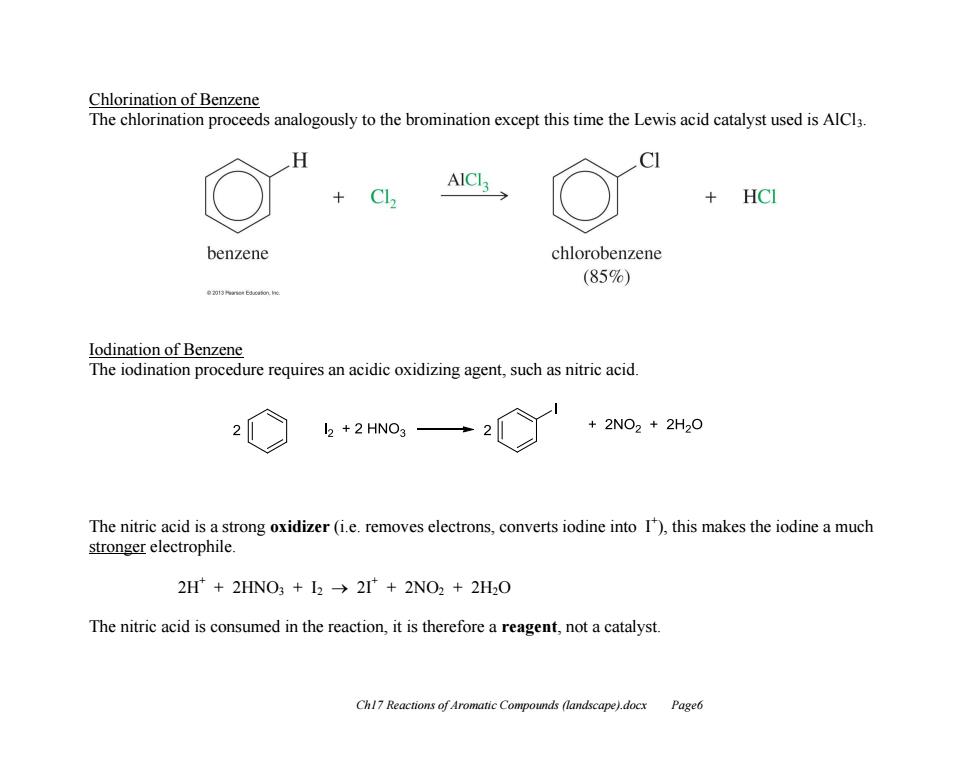
Chlorination of Benzene The chlorination proceeds analogously to the bromination except this time the Lewis acid catalyst used is AlCl3 H AICI HCI benzene chlorobenzene (85%) e23nne6运am.e lodination of Benzene The iodination procedure requires an acidic oxidizing agent,such as nitric acid l2 +2 HNO3 +2NO2+2H20 The nitric acid is a strong oxidizer(i.e.removes electrons,converts iodine into 1),this makes the iodine a much stronger electrophile. 2H+2HNO3 I2 2I+2NO2 +2H2O The nitric acid is consumed in the reaction,it is therefore a reagent,not a catalyst Chl7 Reactions of Aromatic Compounds (landscape).docx Page6
Ch17 Reactions of Aromatic Compounds (landscape).docx Page6 Chlorination of Benzene The chlorination proceeds analogously to the bromination except this time the Lewis acid catalyst used is AlCl3. Iodination of Benzene The iodination procedure requires an acidic oxidizing agent, such as nitric acid. The nitric acid is a strong oxidizer (i.e. removes electrons, converts iodine into I+ ), this makes the iodine a much stronger electrophile. 2H+ + 2HNO3 + I2 2I+ + 2NO2 + 2H2O The nitric acid is consumed in the reaction, it is therefore a reagent, not a catalyst
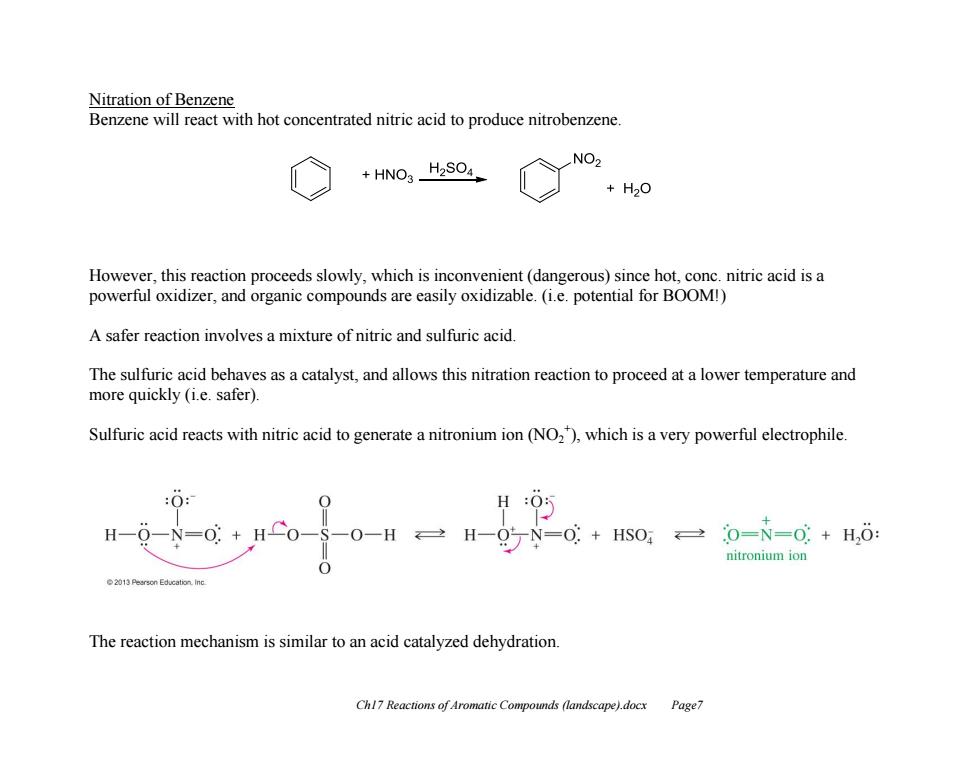
Nitration of Benzene Benzene will react with hot concentrated nitric acid to produce nitrobenzene. HNO3_H2SO4 NO2 +H20 However,this reaction proceeds slowly,which is inconvenient(dangerous)since hot,conc.nitric acid is a powerful oxidizer,and organic compounds are easily oxidizable.(i.e.potential for BOOM!) A safer reaction involves a mixture of nitric and sulfuric acid. The sulfuric acid behaves as a catalyst,and allows this nitration reaction to proceed at a lower temperature and more quickly (i.e.safer). Sulfuric acid reacts with nitric acid to generate a nitronium ion(NO2),which is a very powerful electrophile :O: H H -N=0: 0+HS0,0=N=0+H,: nitronium ion 0 The reaction mechanism is similar to an acid catalyzed dehydration Chl7 Reactions of Aromatic Compounds (landscape).docx Page7
Ch17 Reactions of Aromatic Compounds (landscape).docx Page7 Nitration of Benzene Benzene will react with hot concentrated nitric acid to produce nitrobenzene. However, this reaction proceeds slowly, which is inconvenient (dangerous) since hot, conc. nitric acid is a powerful oxidizer, and organic compounds are easily oxidizable. (i.e. potential for BOOM!) A safer reaction involves a mixture of nitric and sulfuric acid. The sulfuric acid behaves as a catalyst, and allows this nitration reaction to proceed at a lower temperature and more quickly (i.e. safer). Sulfuric acid reacts with nitric acid to generate a nitronium ion (NO2 + ), which is a very powerful electrophile. The reaction mechanism is similar to an acid catalyzed dehydration
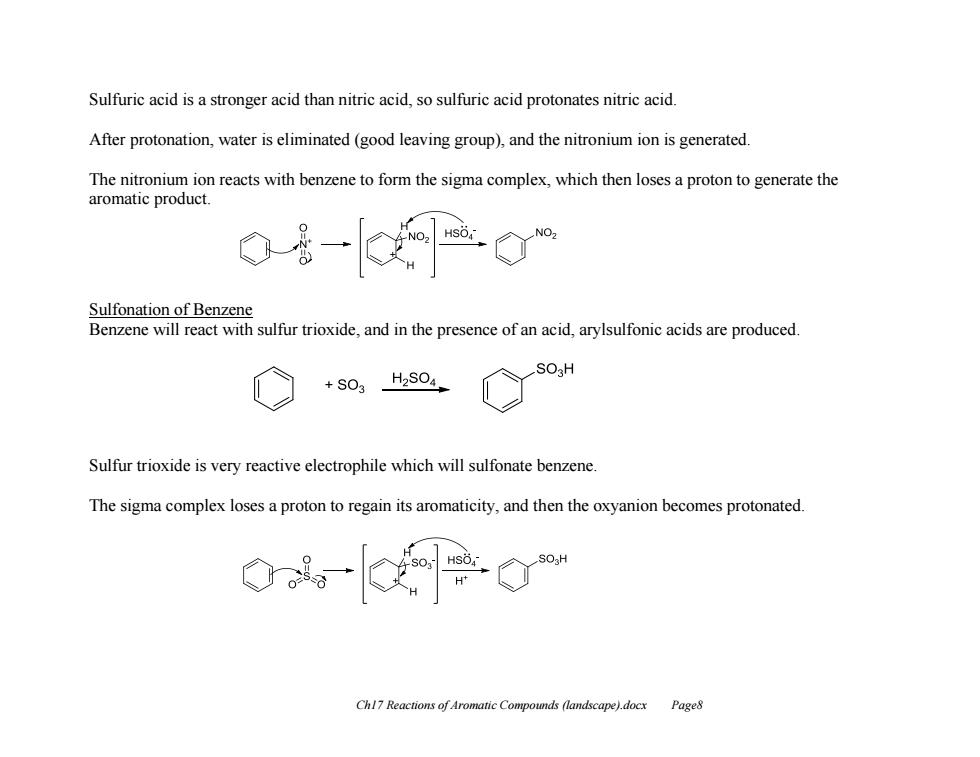
Sulfuric acid is a stronger acid than nitric acid,so sulfuric acid protonates nitric acid. After protonation,water is eliminated (good leaving group),and the nitronium ion is generated The nitronium ion reacts with benzene to form the sigma complex,which then loses a proton to generate the aromatic product. Sulfonation of Benzene Benzene will react with sulfur trioxide,and in the presence of an acid,arylsulfonic acids are produced. +S03 H2S04 Sulfur trioxide is very reactive electrophile which will sulfonate benzene. The sigma complex loses a proton to regain its aromaticity,and then the oxyanion becomes protonated. Chl7 Reactions of Aromatic Compounds (landscape).docx Page8
Ch17 Reactions of Aromatic Compounds (landscape).docx Page8 Sulfuric acid is a stronger acid than nitric acid, so sulfuric acid protonates nitric acid. After protonation, water is eliminated (good leaving group), and the nitronium ion is generated. The nitronium ion reacts with benzene to form the sigma complex, which then loses a proton to generate the aromatic product. Sulfonation of Benzene Benzene will react with sulfur trioxide, and in the presence of an acid, arylsulfonic acids are produced. Sulfur trioxide is very reactive electrophile which will sulfonate benzene. The sigma complex loses a proton to regain its aromaticity, and then the oxyanion becomes protonated
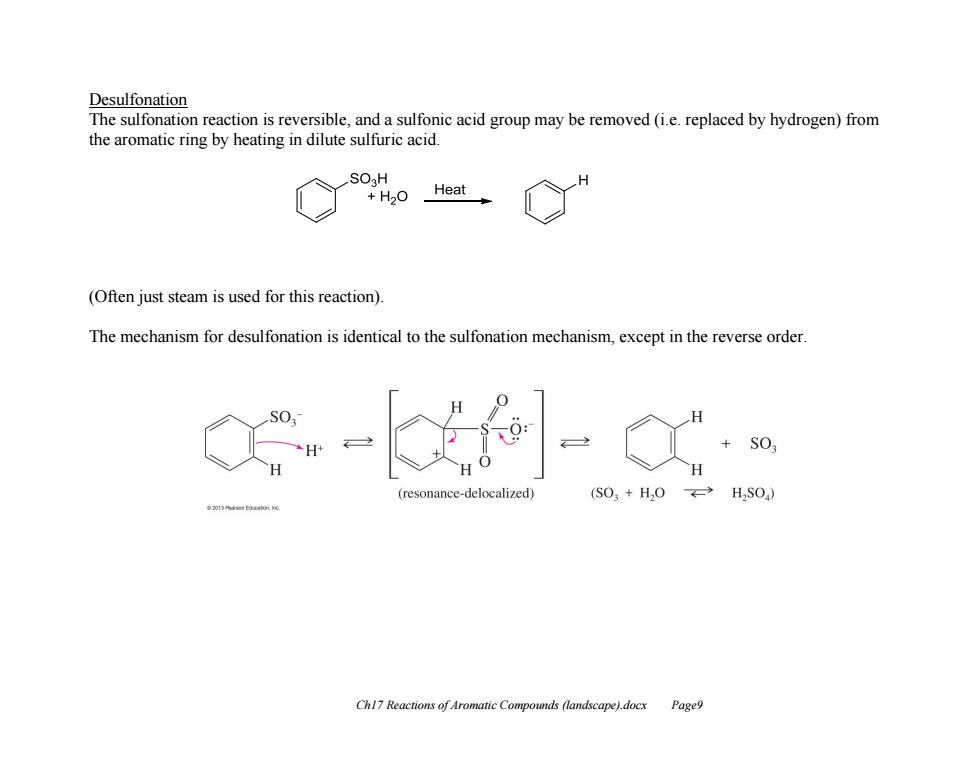
Desulfonation The sulfonation reaction is reversible,and a sulfonic acid group may be removed(i.e.replaced by hydrogen)from the aromatic ring by heating in dilute sulfuric acid. SO H +H20 Heat (Often just steam is used for this reaction) The mechanism for desulfonation is identical to the sulfonation mechanism,except in the reverse order SO ce-delocalized) (S03+H,0←-HSO) Chl7 Reactions of Aromatic Compounds (landscape).docx Page9
Ch17 Reactions of Aromatic Compounds (landscape).docx Page9 Desulfonation The sulfonation reaction is reversible, and a sulfonic acid group may be removed (i.e. replaced by hydrogen) from the aromatic ring by heating in dilute sulfuric acid. (Often just steam is used for this reaction). The mechanism for desulfonation is identical to the sulfonation mechanism, except in the reverse order

Hydrogen-Deuterium Exchange Protonation of the benzene ring may also occur by this mechanism (D,0) (resonance-delocalized) After protonation has occurred,the sigma complex can lose either of the hydrogens from the sp'carbon to regain its aromaticity To prove that reaction has actually occurred,deuterated sulfuric acid can be used. The products will have deuterium substituted for hydrogen. If a large excess of deuterated reagent is used,hexadeuteriobenzene can be produced from this equilibrium reaction. Chl7 Reactions of Aromatic Compounds (landscape).docx Pagel0
Ch17 Reactions of Aromatic Compounds (landscape).docx Page10 Hydrogen-Deuterium Exchange Protonation of the benzene ring may also occur by this mechanism. After protonation has occurred, the sigma complex can lose either of the hydrogens from the sp3 carbon to regain its aromaticity. To prove that reaction has actually occurred, deuterated sulfuric acid can be used. The products will have deuterium substituted for hydrogen. If a large excess of deuterated reagent is used, hexadeuteriobenzene can be produced from this equilibrium reaction