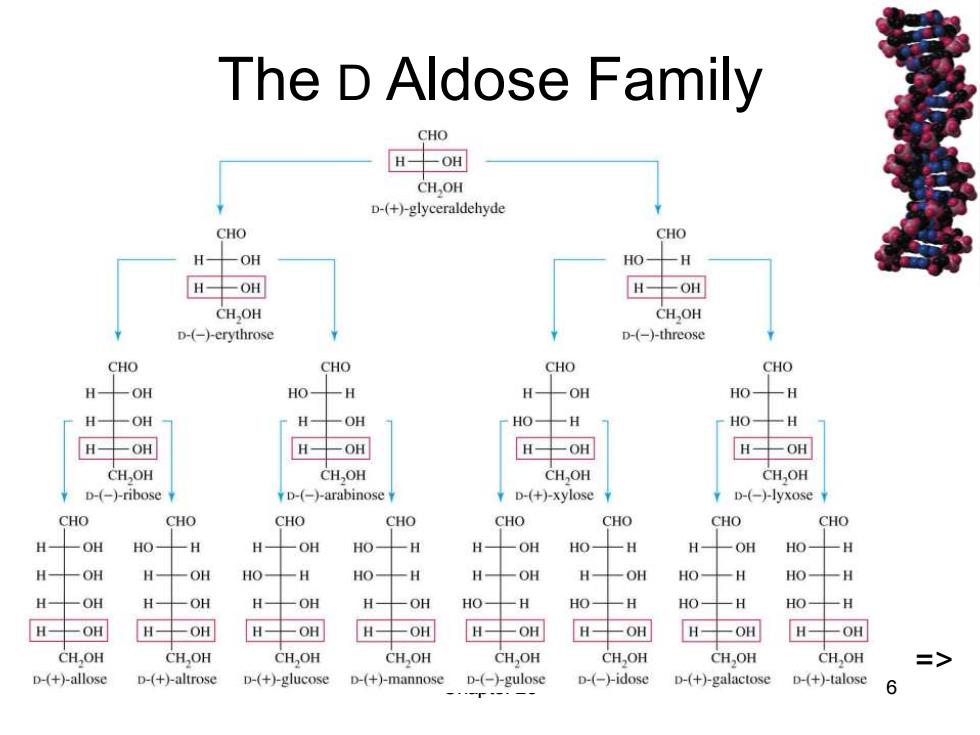
The D Aldose Family CHO HOH CH OH D-(+)-glyceraldehyde CHO CHO H-OH HO十H H生OH H-OH CHOH CH,OH D-(-)erythrose D-(-)-threose CHO CHO CHO CHO H-OH HO- -H H-OH HO- 一H H-OH H- 一OH HO- 一H HO- -H H-OH H-OH H-OH H-OH CHOH CH,OH CHOH CH,OH ¥D-(-)-ribose D-(-)-arabinose D-(+)-xylose D-(-)-lyxose CHO CHO CHO CHO CHO CHO CHO CHO H-OH HO- 一H H- -OH HO- -H H -OH HO- 一H H -OH HO-H H-OH H- OH HO- 一H HO- -H H- 一OH H -OH HO- 一H HO-H H-OH H-OH H 一OH H- -OH HO- 一H H0一 一H HO- -H HO-H H-OH H-OH H-OH H-OH H-OH H-OH H-OH H-OH CH,OH CH,OH CH,OH CH,OH CH,OH CHOH CH,OH CHOH => D-(+)-allose D-(+)-altrose D-(+)-glucose D-(+)-mannose D-(-)-gulose D-(-)-idose D-(+)-galactose D-(+)-talose 6
Chapter 23 6 The D Aldose Family =>
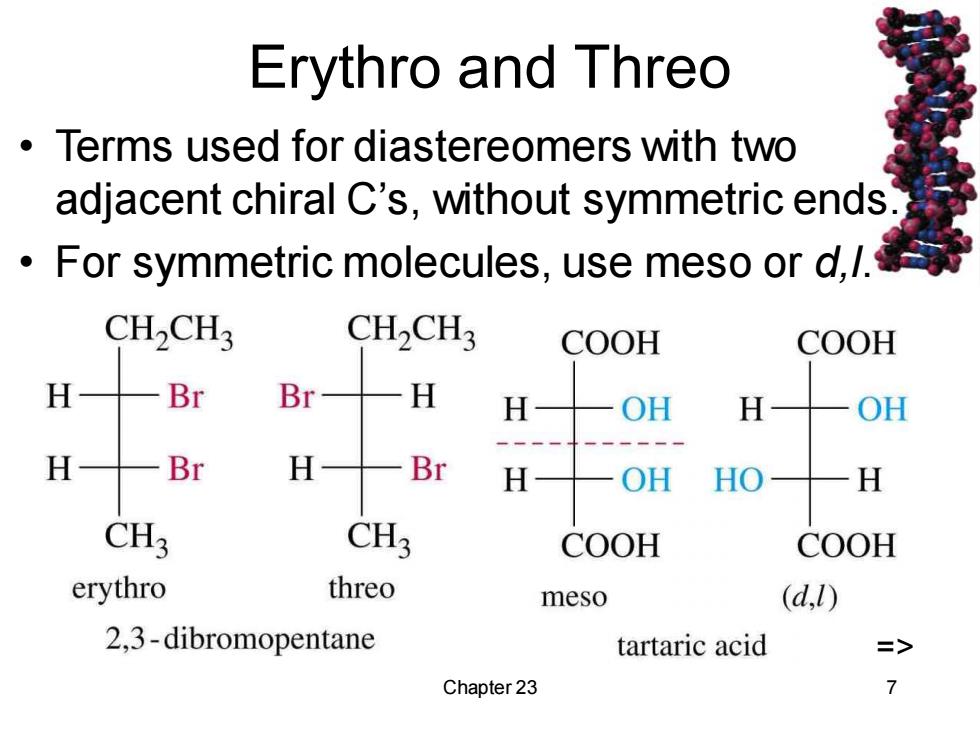
Erythro and Threo Terms used for diastereomers with two adjacent chiral C's,without symmetric ends For symmetric molecules,use meso or d,/. CH2CH3 CH2CH3 COOH COOH H Br Br H H OH H OH H Br H Br H OH HO H CH3 CH3 COOH COOH erythro threo meso (d,l) 2,3-dibromopentane tartaric acid => Chapter 23 7
Chapter 23 7 Erythro and Threo • Terms used for diastereomers with two adjacent chiral C’s, without symmetric ends. • For symmetric molecules, use meso or d,l. =>
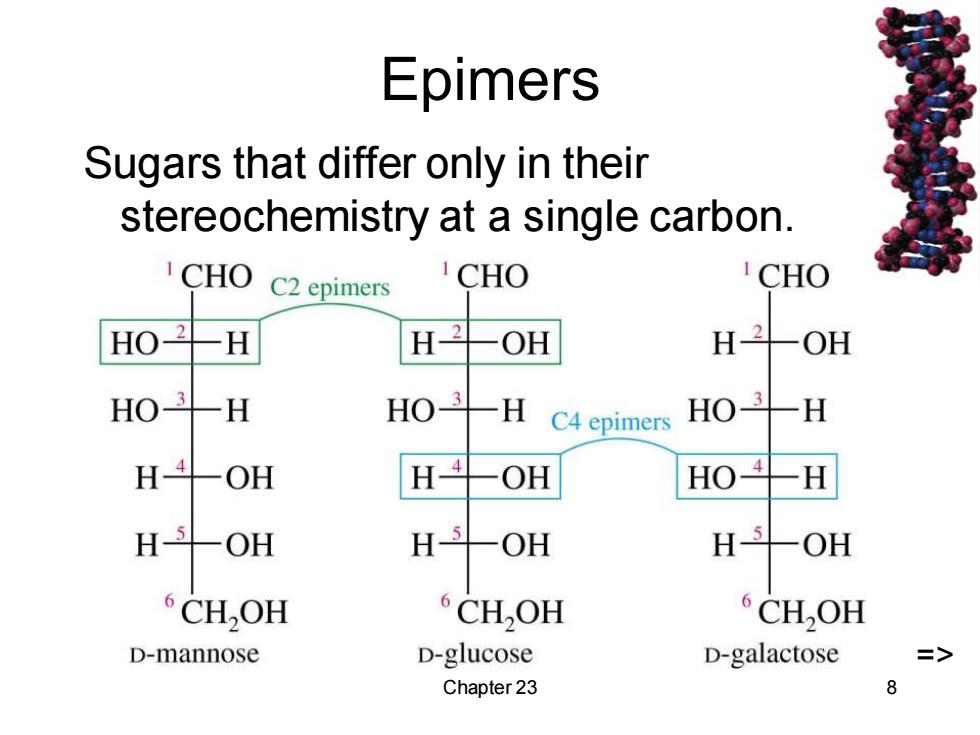
Epimers Sugars that differ only in their stereochemistry at a single carbon. 1CHO C2epimers CHO CHO H H-2 OH H-2 一 OH HO3H HO-3H C4 epimers HO3H H-4-OH H-4-OH HO牛H H-5-OH H-5-OH H CH,OH CH,OH CH,OH D-mannose D-glucose D-galactose => Chapter 23 8
Chapter 23 8 Epimers Sugars that differ only in their stereochemistry at a single carbon. =>
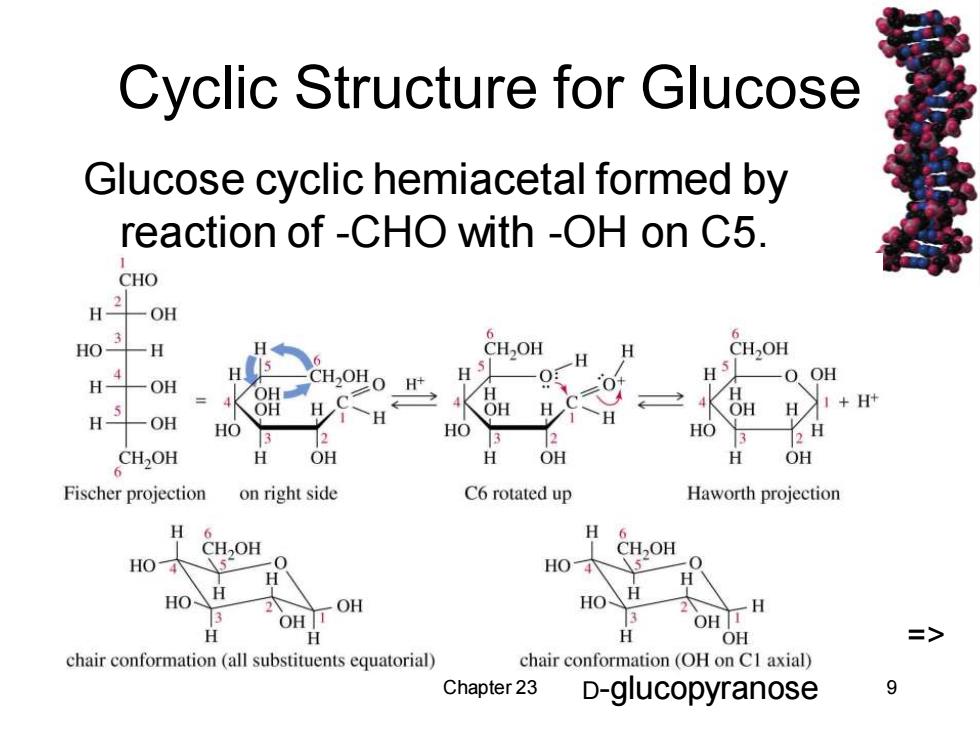
Cyclic Structure for Glucose Glucose cyclic hemiacetal formed by reaction of -CHO with -OH on C5. CHO H 一OH HO- -H CH,OH H CHOH 4 H OH H -OH CH-OH O H+ OH H OH H OH H +H+ H OH HO HO HO 2 CH2OH H OH OH OH Fischer projection on right side C6 rotated up Haworth projection H 6 H 6 CHOH CH,OH 5 HO 5■ HO OH 2 -H 3 OH T OH 分 H OH => chair conformation(all substituents equatorial) chair conformation(OH on CI axial) Chapter 23 D-glucopyranose 9
Chapter 23 9 Cyclic Structure for Glucose Glucose cyclic hemiacetal formed by reaction of -CHO with -OH on C5. => D-glucopyranose
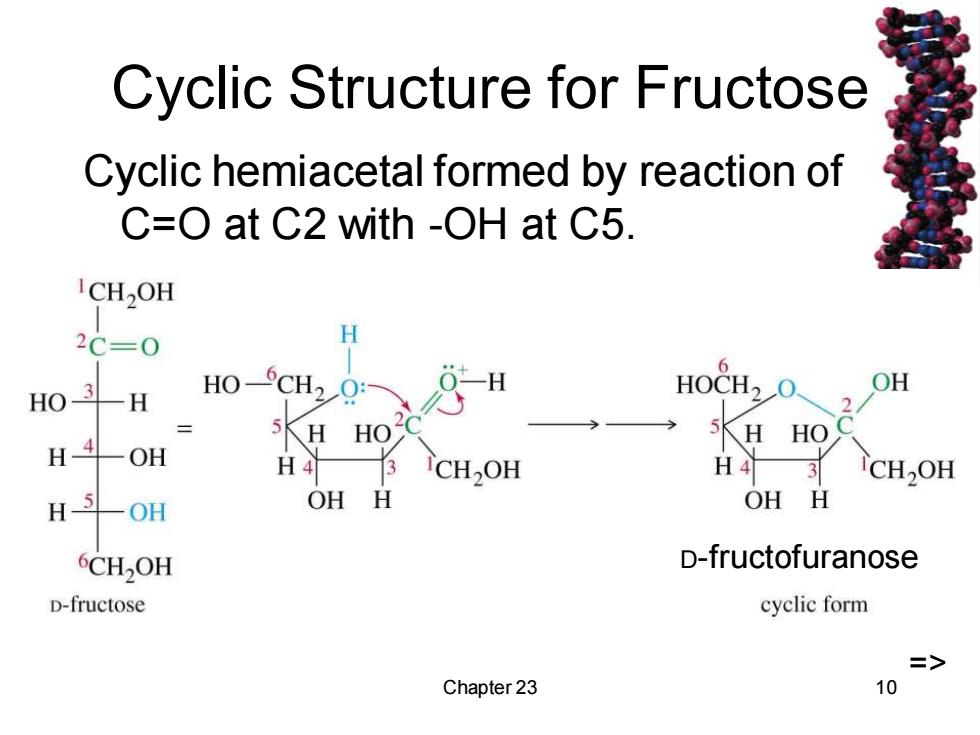
Cyclic Structure for Fructose Cyclic hemiacetal formed by reaction of C=O at C2 with -OH at C5. ICH2OH 2C=0 H HO-CH2Q: 0-H 6 HO3-H OH 2 HO H4-OH 5K日 HO H4 3 H4 3 CH2OH H 一OH OH H OH H 6CH2OH D-fructofuranose D-fructose cyclic form => Chapter 23 10
Chapter 23 10 Cyclic Structure for Fructose Cyclic hemiacetal formed by reaction of C=O at C2 with -OH at C5. => D-fructofuranose