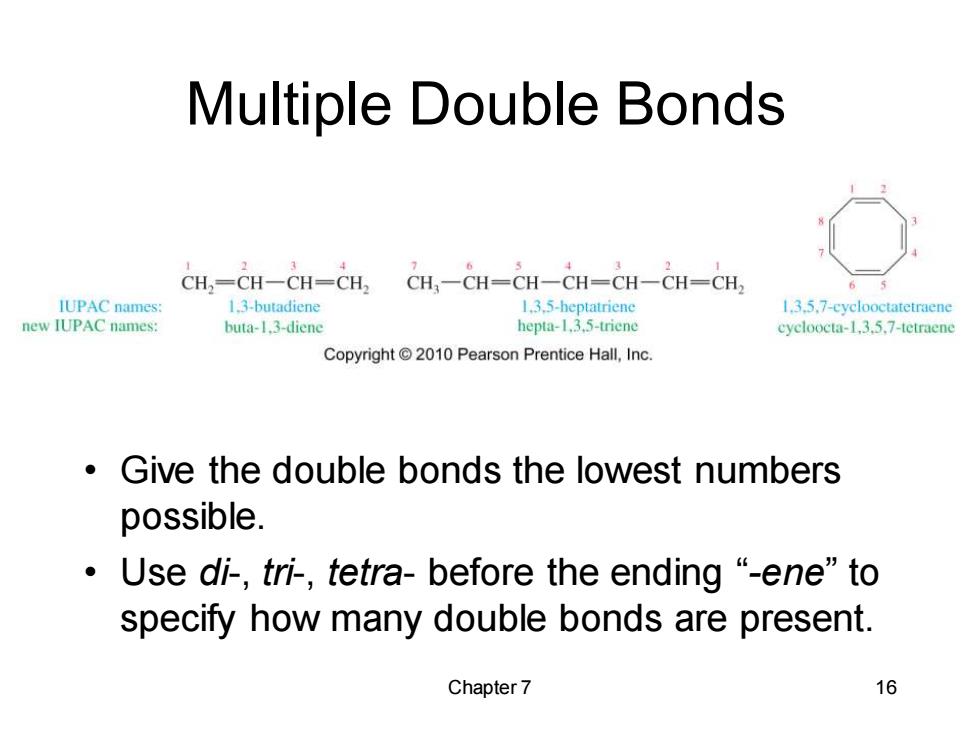
Multiple Double Bonds CH,=CH一CH=CH2 CH,-CH-CH-CH-CH-CH-CH IUPAC names 1.3-butadiene 1.3.5-heptatriene 1.3.5.7-cyclooctatetraene new IUPAC names: buta-1.3-diene hepta-1,3.5-triene cycloocta-13.5.7-tetraene Copyright 2010 Pearson Prentice Hall,Inc. Give the double bonds the lowest numbers possible. ·Use di-,tri,tetra-before the ending“-ene”to specify how many double bonds are present. Chapter 7 16
Chapter 7 16 Multiple Double Bonds • Give the double bonds the lowest numbers possible. • Use di-, tri-, tetra- before the ending “-ene” to specify how many double bonds are present
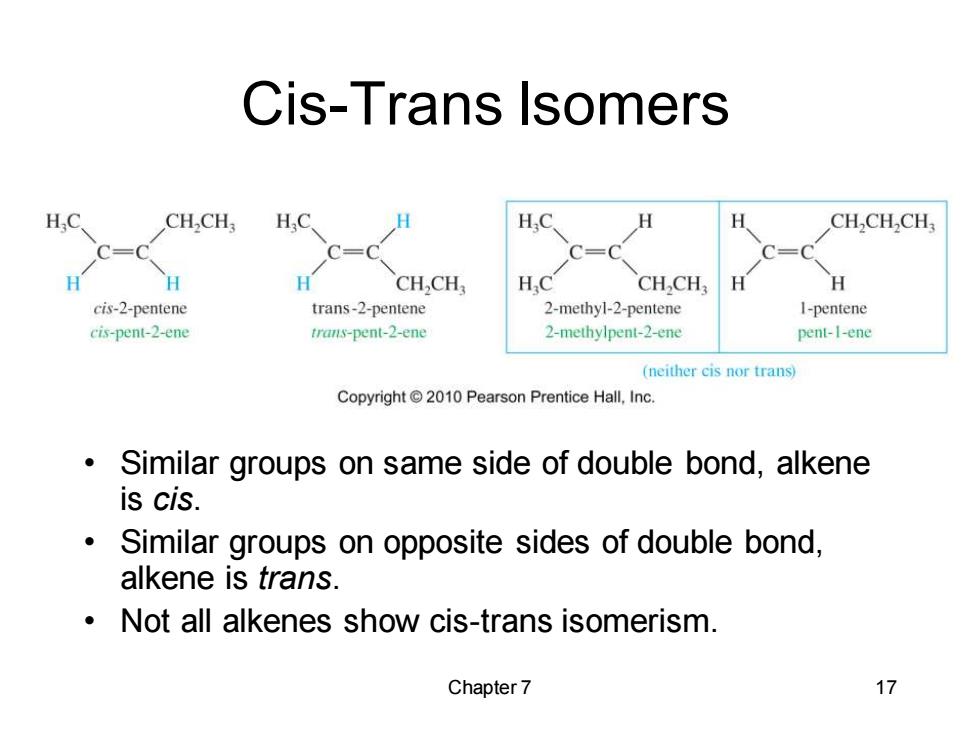
Cis-Trans lsomers H.C CHCH: H.C. H.O H CHCH2CH C= H H CH2CH HC CHCH H H cis-2-pentene trans-2-pentene 2-methyl-2-pentene I-pentene cis-pent-2-ene trans-pent-2-ene 2-methylpent-2-ene pent-1-ene (neither cis nor trans) Copyright2010 Pearson Prentice Hall,Inc. Similar groups on same side of double bond,alkene is cis. Similar groups on opposite sides of double bond, alkene is trans. Not all alkenes show cis-trans isomerism. Chapter 7 17
Chapter 7 17 Cis-Trans Isomers • Similar groups on same side of double bond, alkene is cis. • Similar groups on opposite sides of double bond, alkene is trans. • Not all alkenes show cis-trans isomerism