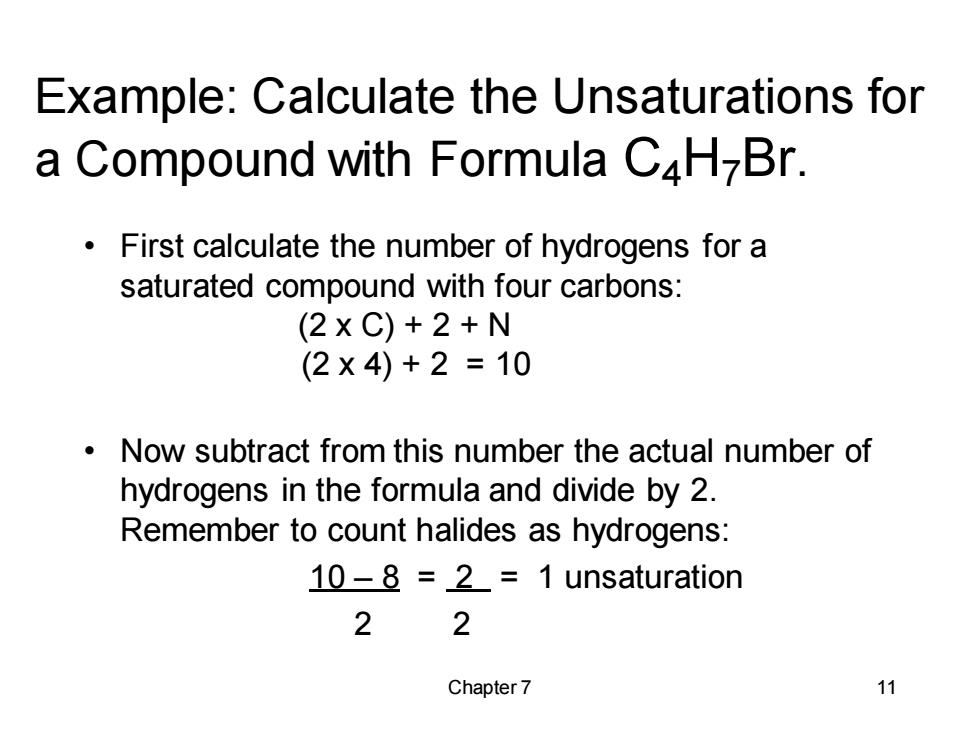
Example:Calculate the Unsaturations for a Compound with Formula C4H-Br. First calculate the number of hydrogens for a saturated compound with four carbons: (2xC)+2+N (2×4)+2=10 Now subtract from this number the actual number of hydrogens in the formula and divide by 2. Remember to count halides as hydrogens: 10-8 =2=1 unsaturation 2 2 Chapter 7 11
Chapter 7 11 Example: Calculate the Unsaturations for a Compound with Formula C4H7Br. • First calculate the number of hydrogens for a saturated compound with four carbons: (2 x C) + 2 + N (2 x 4) + 2 = 10 • Now subtract from this number the actual number of hydrogens in the formula and divide by 2. Remember to count halides as hydrogens: 10 – 8 = 2 = 1 unsaturation 2 2
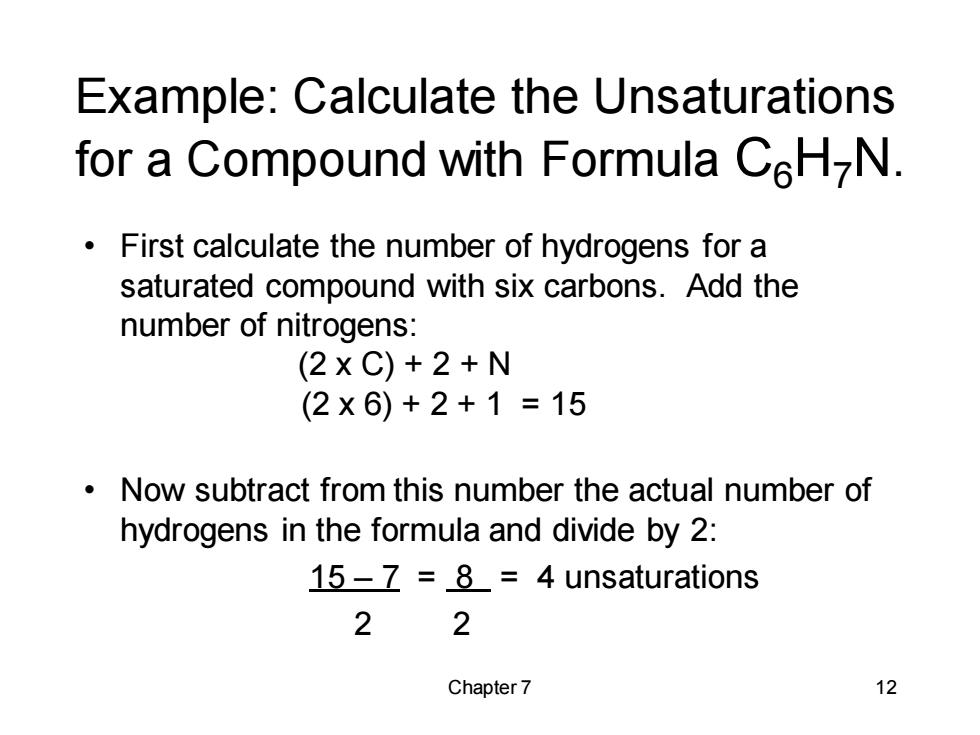
Example:Calculate the Unsaturations for a Compound with Formula CH-N. First calculate the number of hydrogens for a saturated compound with six carbons.Add the number of nitrogens: (2xC)+2+N (2×6)+2+1=15 Now subtract from this number the actual number of hydrogens in the formula and divide by 2: 15-7 =8=4 unsaturations 2 2 Chapter 7 12
Chapter 7 12 Example: Calculate the Unsaturations for a Compound with Formula C6H7N. • First calculate the number of hydrogens for a saturated compound with six carbons. Add the number of nitrogens: (2 x C) + 2 + N (2 x 6) + 2 + 1 = 15 • Now subtract from this number the actual number of hydrogens in the formula and divide by 2: 15 – 7 = 8 = 4 unsaturations 2 2

IUPAC Nomenclature Find the longest continuous carbon chain that includes the double-bonded carbons. 。-ane changes to-ene. Number the chain so that the double bond has the lowest possible number. In a ring,the double bond is assumed to be between Carbon 1 and Carbon 2. Chapter 7 13
Chapter 7 13 IUPAC Nomenclature • Find the longest continuous carbon chain that includes the double-bonded carbons. • -ane changes to -ene. • Number the chain so that the double bond has the lowest possible number. • In a ring, the double bond is assumed to be between Carbon 1 and Carbon 2
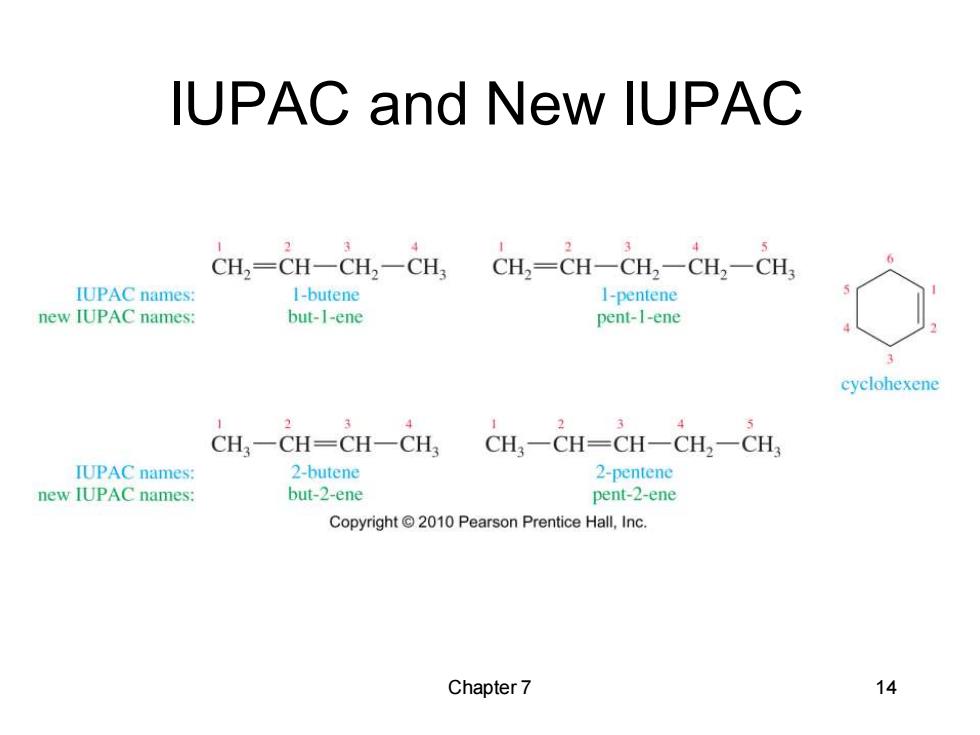
IUPAC and New IUPAC CH2=CH-CH2一CH CH2=CH-CH2一CH2-CH IUPAC names: 1-butene 1-pentene new IUPAC names: but-1-ene pent-I-ene cyclohexene 2 2 CH一CH=CH-CH CH3一CH=CH一CH2一CH IUPAC names: 2-butene 2-pentene new IUPAC names: but-2-ene pent-2-ene Copyright 2010 Pearson Prentice Hall,Inc. Chapter 7 14
Chapter 7 14 IUPAC and New IUPAC

Ring Nomenclature In a ring,the double bond is assumed to be between Carbon 1 and Carbon 2. CH3 3 CH3 2 1-methylcyclopentene 3-methylcyclopentene Chapter 7 15
Chapter 7 15 Ring Nomenclature 1-methylcyclopentene In a ring, the double bond is assumed to be between Carbon 1 and Carbon 2. CH3 CH3 1 2 1 2 3 3-methylcyclopentene