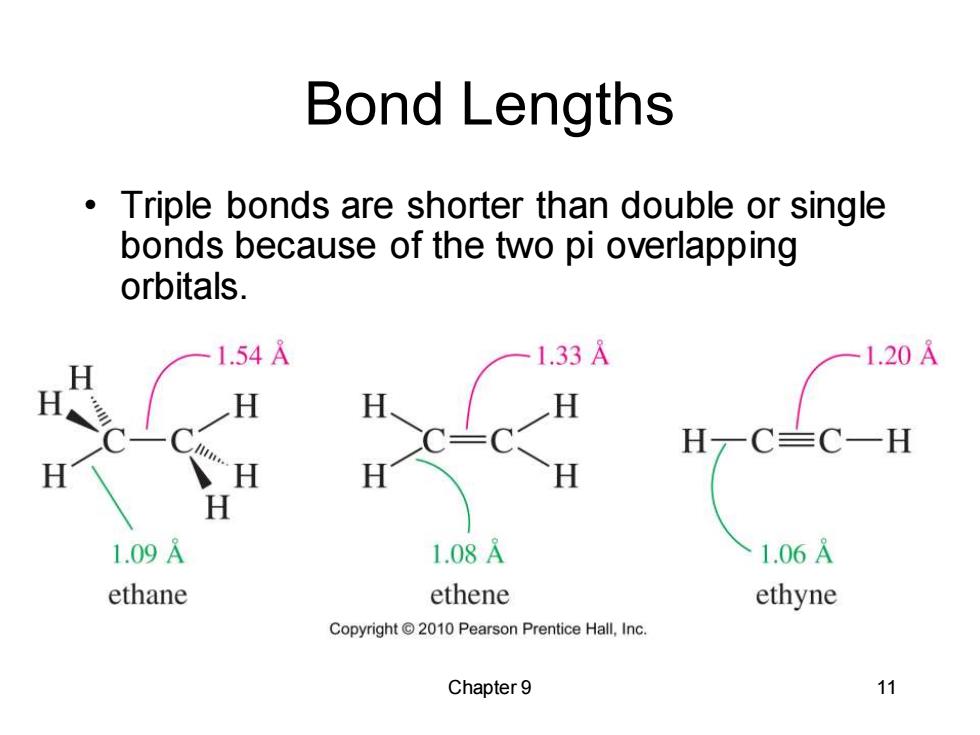
Bond Lengths Triple bonds are shorter than double or single bonds because of the two pi overlapping orbitals. 1.54A 1.33A 1.20A H H-c HC=C一H H H H 1.09A 1.08A 1.06A ethane ethene ethyne Copyright 2010 Pearson Prentice Hall,Inc. Chapter 9 11
Chapter 9 11 Bond Lengths • Triple bonds are shorter than double or single bonds because of the two pi overlapping orbitals
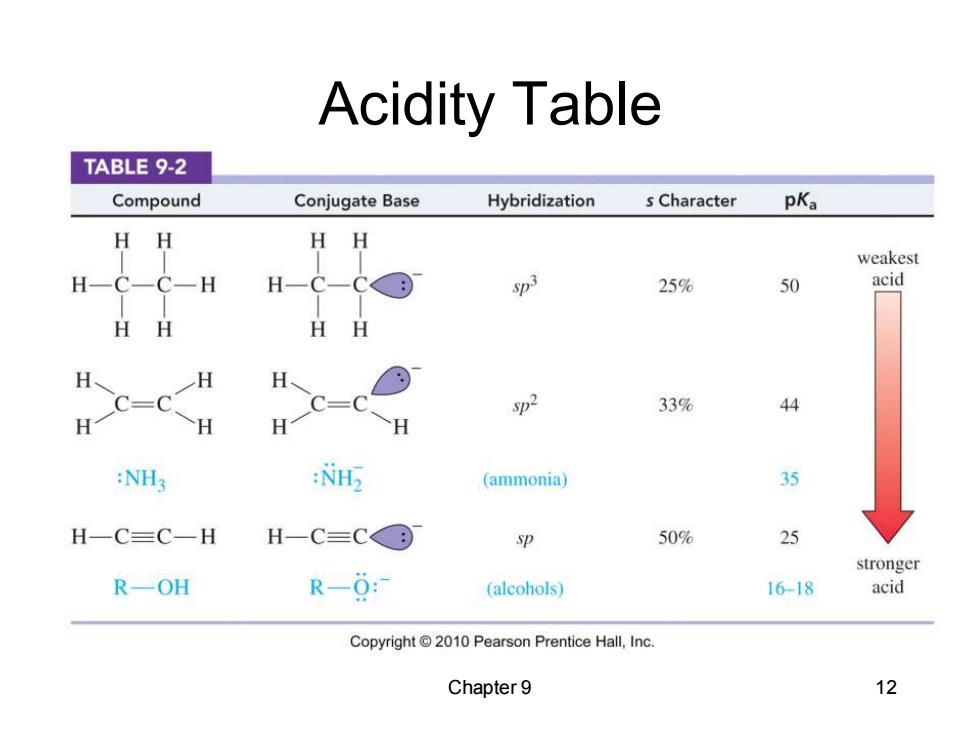
Acidity Table TABLE 9-2 Compound Conjugate Base Hybridization s Character pKa H H HH weakest H C-C-H H-C-C D: 25% 50 acid HH HH H H H C=C sp2 33% 44 H H H H :NH3 : (ammonia) 35 H一C三C一H H-C=C© sp 50% 25 stronger R一OH R-0 (alcohols) 16-18 acid Copyright2010 Pearson Prentice Hall,Inc. Chapter 9 12
Chapter 9 12 Acidity Table

Acidity of Alkynes Terminal alkynes,are more acidic than other hydrocarbons due to the higher s character of the sp hybridized carbon. Terminal alkynes can be deprotonated quantitatively with strong bases such as sodium amide (NH2). Hydroxide and alkoxide bases are not strong enough to deprotonate the alkyne quantitatively. Chapter 9 13
Chapter 9 13 Acidity of Alkynes • Terminal alkynes, are more acidic than other hydrocarbons due to the higher s character of the sp hybridized carbon. • Terminal alkynes can be deprotonated quantitatively with strong bases such as sodium amide (-NH2). • Hydroxide and alkoxide bases are not strong enough to deprotonate the alkyne quantitatively
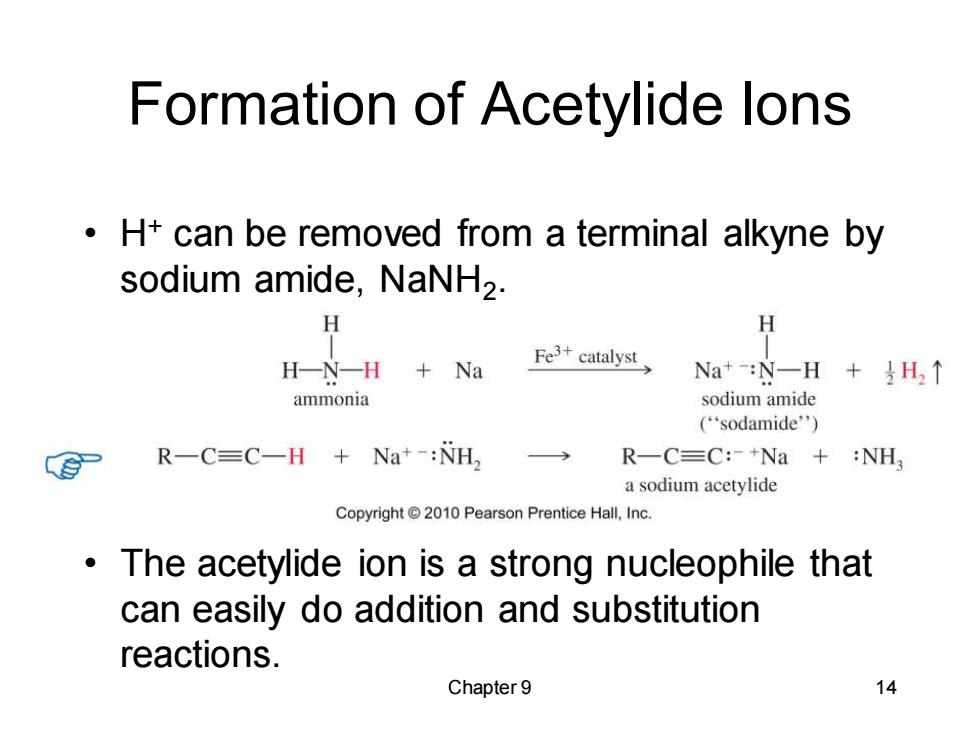
Formation of Acetylide lons H+can be removed from a terminal alkyne by sodium amide,NaNH2. H H-N-H Na Fe3+catalyst Na+:N一H+H↑ ammonia sodium amide (“sodamide'') R-C=C-H Na+-:NH2 R一C=C:+Na+:NH, a sodium acetylide Copyright 2010 Pearson Prentice Hall,Inc. The acetylide ion is a strong nucleophile that can easily do addition and substitution reactions. Chapter 9 14
Chapter 9 14 Formation of Acetylide Ions • H+ can be removed from a terminal alkyne by sodium amide, NaNH2 . • The acetylide ion is a strong nucleophile that can easily do addition and substitution reactions
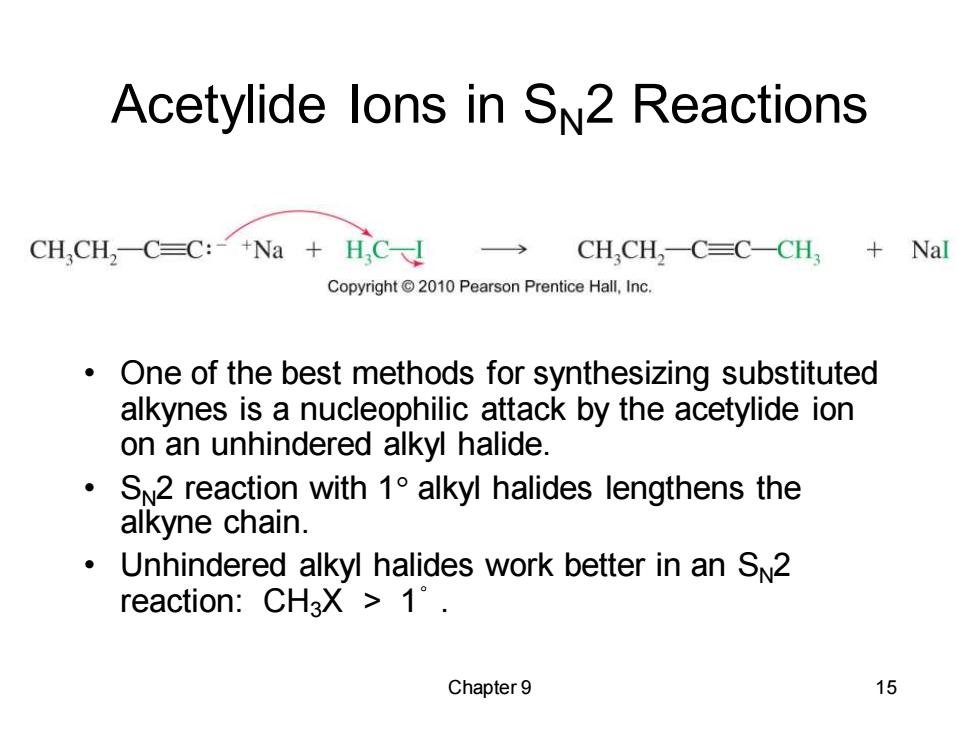
Acetylide lons in SN2 Reactions CH,CH,-C=C:-+Na H;CI CH,CH2-C=C-CH,Nal Copyright2010 Pearson Prentice Hall,Inc. One of the best methods for synthesizing substituted alkynes is a nucleophilic attack by the acetylide ion on an unhindered alkyl halide. 。 SN2 reaction with 1 alkyl halides lengthens the alkyne chain. Unhindered alkyl halides work better in an SN2 reaction:CH3X>1°. Chapter9 15
Chapter 9 15 Acetylide Ions in SN2 Reactions • One of the best methods for synthesizing substituted alkynes is a nucleophilic attack by the acetylide ion on an unhindered alkyl halide. • SN2 reaction with 1 alkyl halides lengthens the alkyne chain. • Unhindered alkyl halides work better in an SN2 reaction: CH3X > 1°