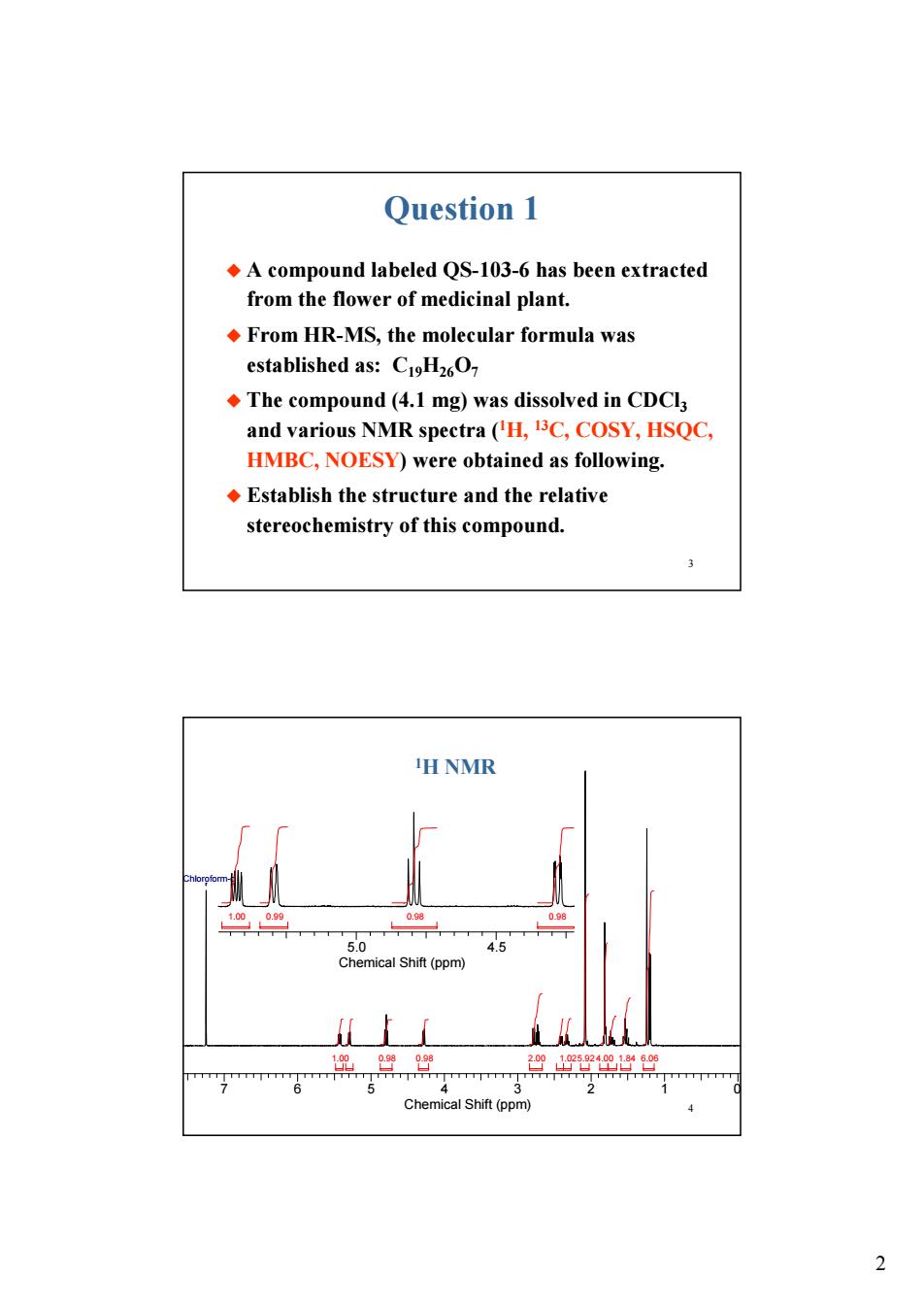
Question 1 A compound labeled QS-103-6 has been extracted from the flower of medicinal plant. From HR-MS,the molecular formula was established as:C1H26O The compound(4.1 mg)was dissolved in CDCla and various NMR speetra (H,13C,COSY,HSQC. HMBC,NOESY)were obtained as following. Establish the structure and the relative stereochemistry of this compound. H NMR 45 ical Shift (ppm) 2
2 3 Question 1 u A compound labeled QS-103-6 has been extracted from the flower of medicinal plant. u From HR-MS, the molecular formula was established as: C19H26O7 u The compound (4.1 mg) was dissolved in CDCl3 and various NMR spectra (1H, 13C, COSY, HSQC, HMBC, NOESY) were obtained as following. u Establish the structure and the relative stereochemistry of this compound. 4 7 6 5 4 3 2 1 0 Chemical Shift (ppm) 1.00 0.98 0.98 2.00 1.025.92 4.00 1.84 6.06 Chloroform-d 5.0 4.5 Chemical Shift (ppm) 1.00 0.99 0.98 0.98 1H NMR
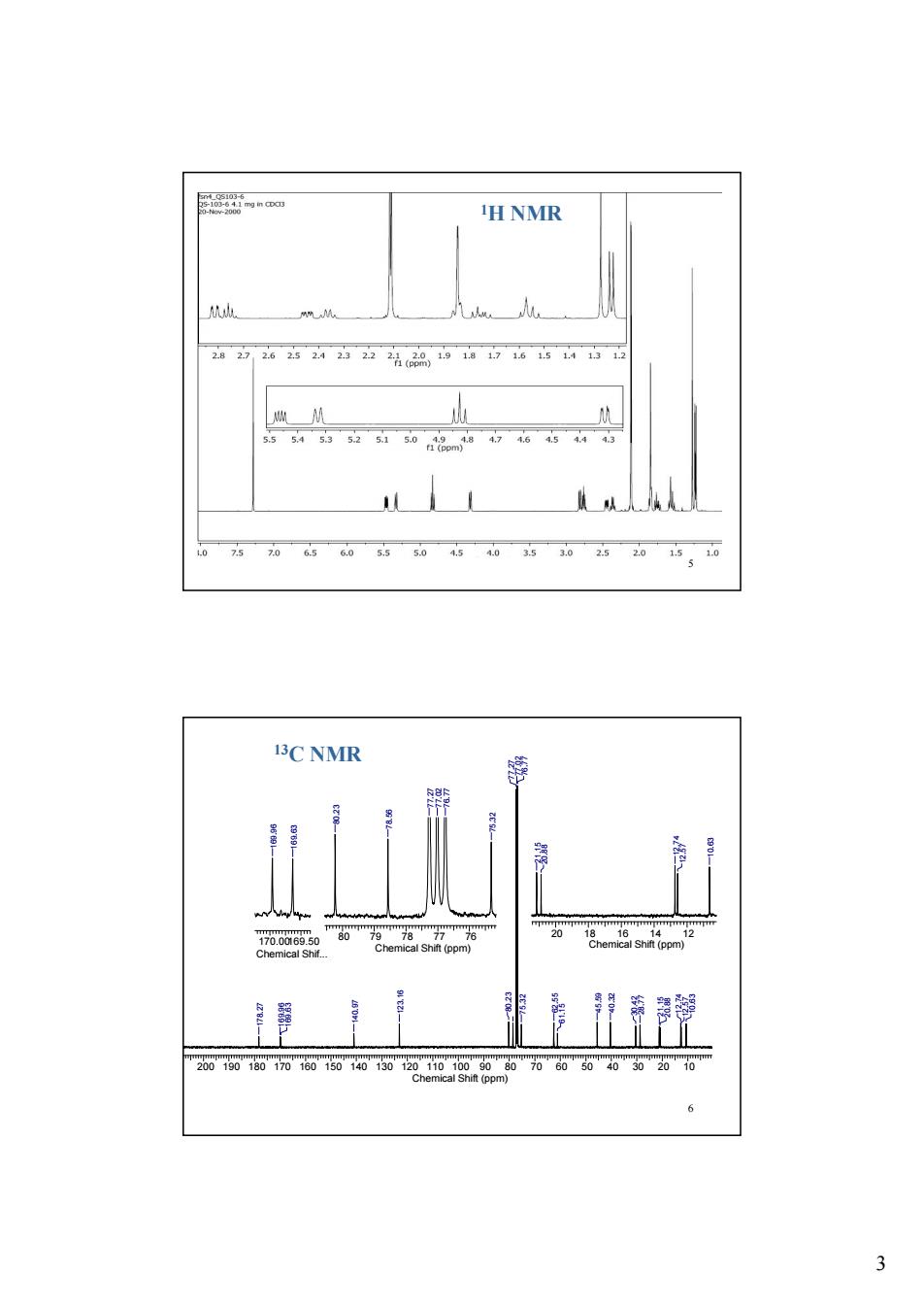
H NMR 282262524232222品19116154112 1 5.5 C NMR 力gem8spm2 20的00050w0200000的020动260 3
3 5 1H NMR 6 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 Chemical Shift (ppm) 178.27 169.96 169.63 140.97 123.16 80.23 77.27 77.02 76.77 75.32 62.55 61.15 45.59 40.32 30.42 28.77 21.15 20.88 12.74 12.57 10.63 170.00169.50 Chemical Shif... 169.96 169.63 80 79 78 77 76 Chemical Shift (ppm) 80.23 78.56 77.27 77.02 76.77 75.32 20 18 16 14 12 Chemical Shift (ppm) 21.15 20.88 12.74 12.57 10.63 13C NMR
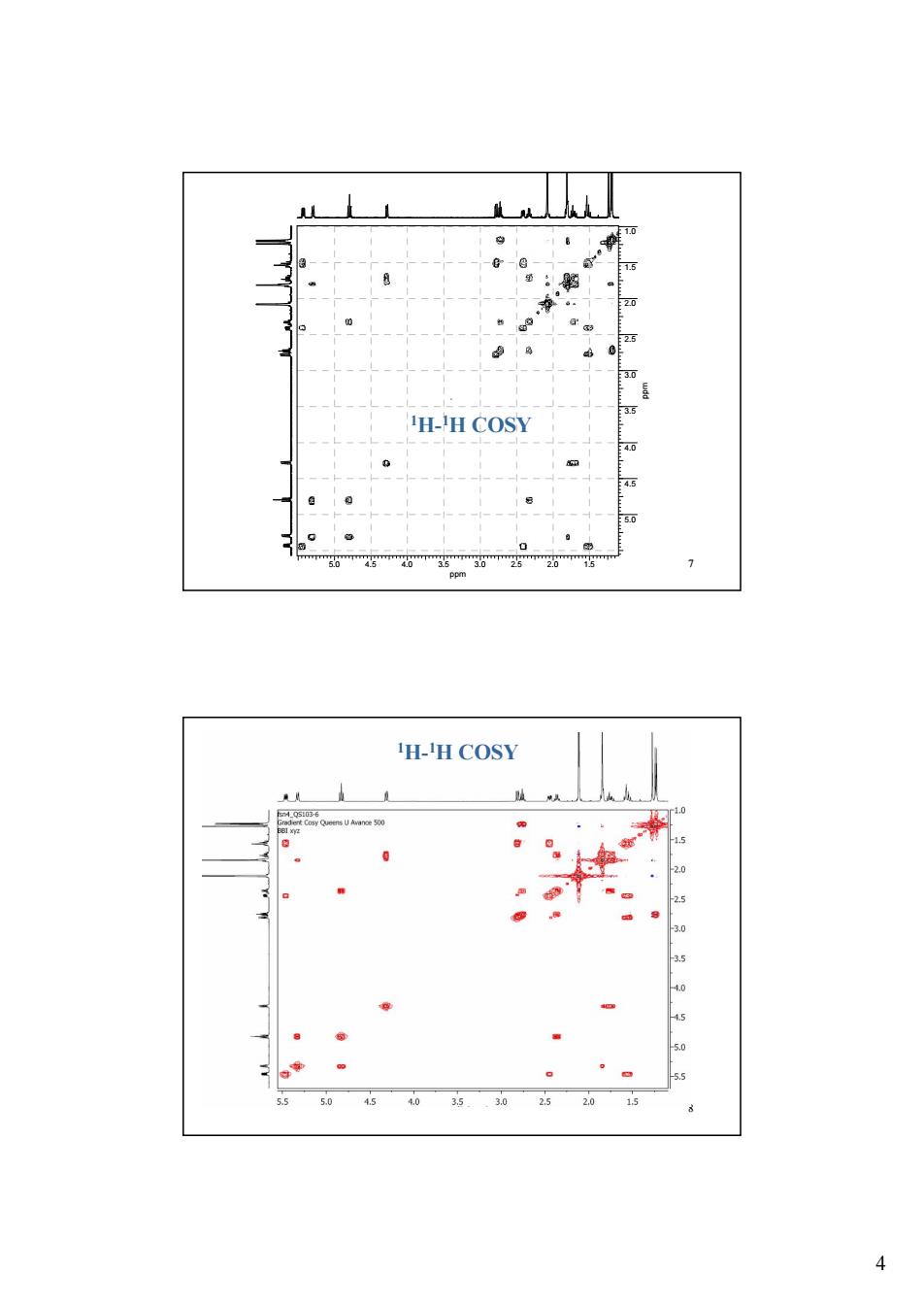
H-H COSY 西 。 H-H COSY · 5 25 45 55 505035302620
4 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 7 ppm 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 ppm 1H-1H COSY 8 1H-1H COSY

o 3030462983030262218w HMBC 50 6303302622181 5
5 9 HSQC 10 HMBC
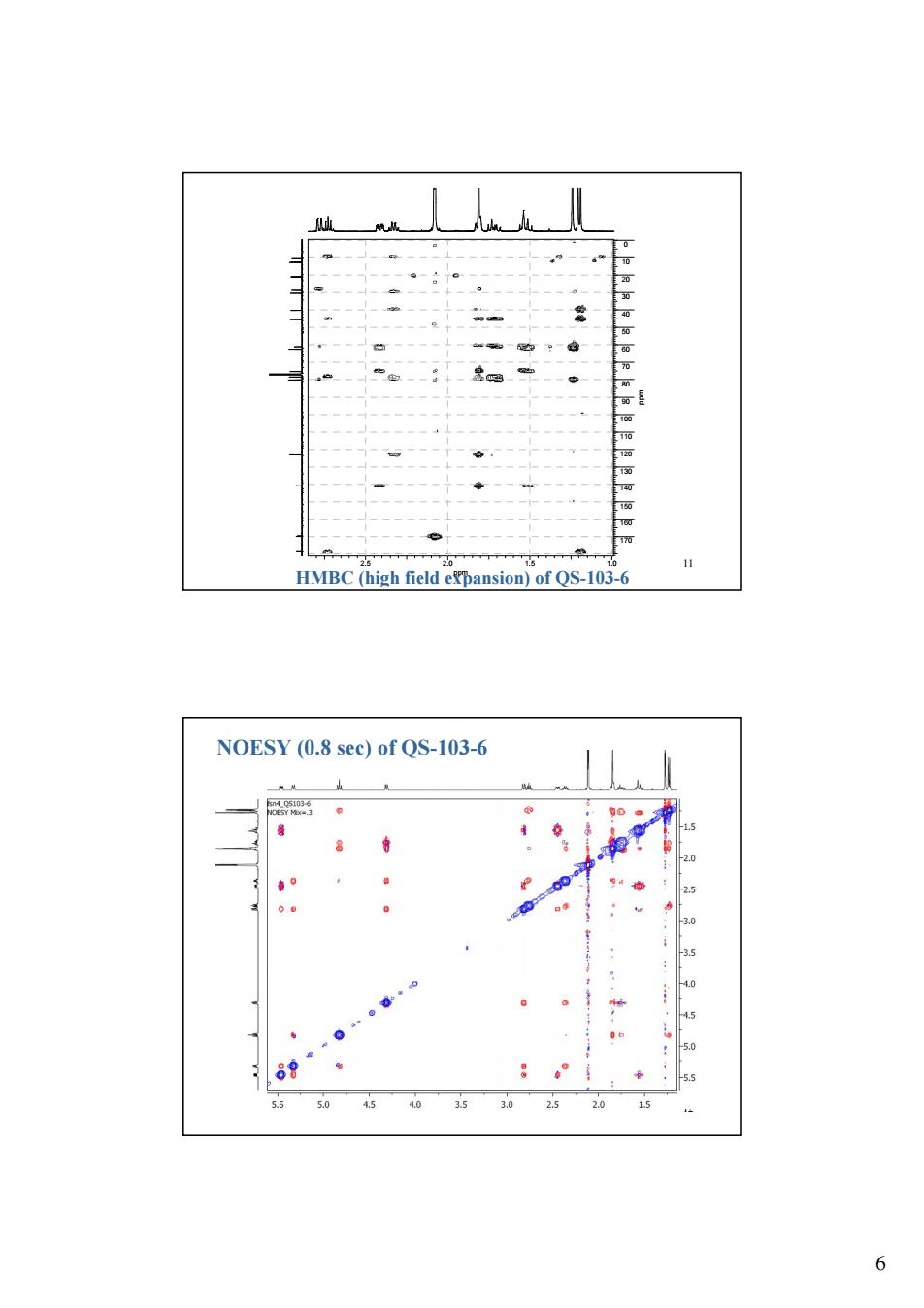
11 HMBC (high field expansion)of QS-103-6 NOESY (0.8 sec)of QS-103-6 3.0 3.5 80 5.5 45 30 6
6 11 HMBC (high field expansion) of QS-103-6 2.5 2.0 1.5 1.0 ppm 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 ppm 12 NOESY (0.8 sec) of QS-103-6