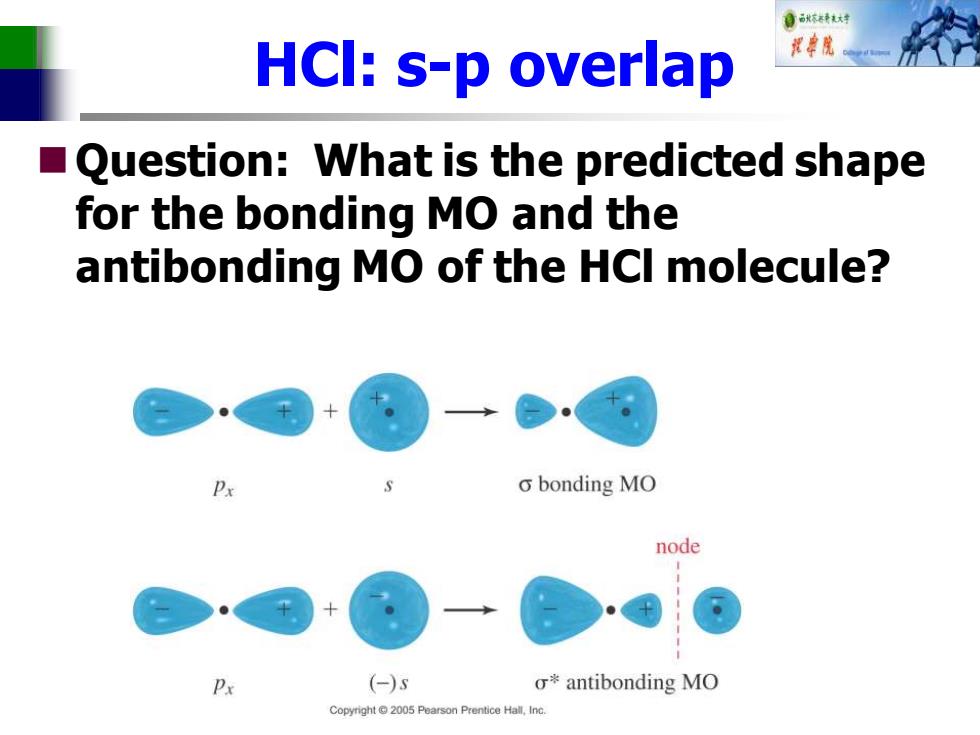
自秋不特大对 HCI:s-p overlap ■( Question:What is the predicted shape for the bonding MO and the antibonding MO of the HCI molecule? o bonding MO node Px (-)s σ*antibonding MO Copyright 2005 Pearson Prentice Hall,Inc
HCl: s-p overlap ◼Question: What is the predicted shape for the bonding MO and the antibonding MO of the HCl molecule?
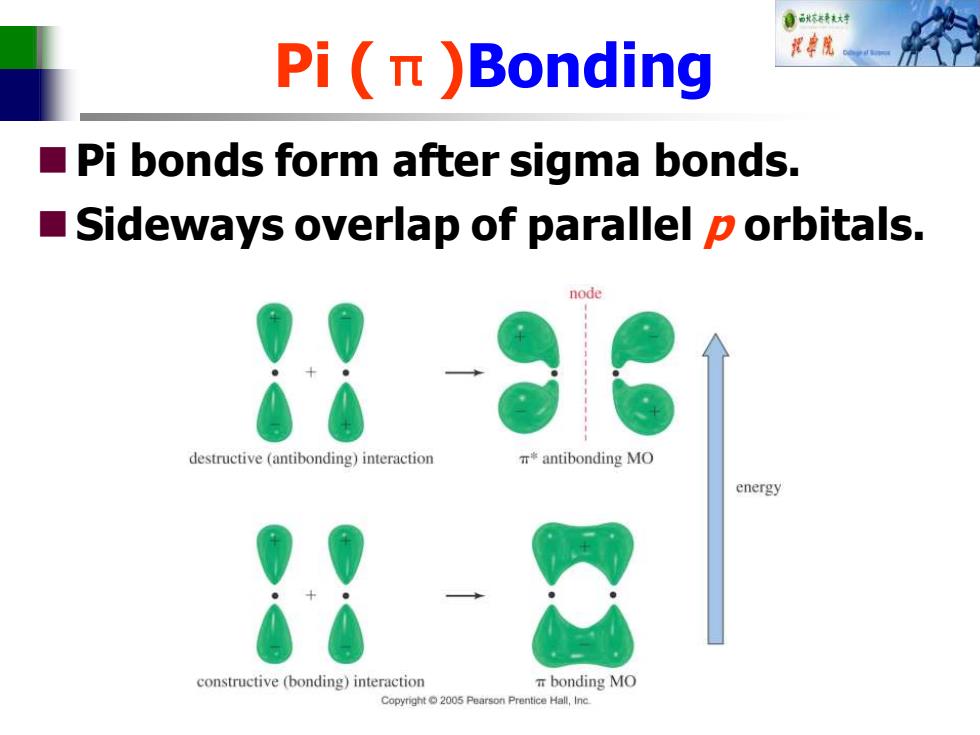
自标特大对 Pi(n )Bonding ■ Pi bonds form after sigma bonds. ■ Sideways overlap of parallel p orbitals. node destructive(antibonding)interaction antibonding MO energy constructive (bonding)interaction T bonding MO Copyright2005 Pearson Prentice Hall,Inc
Pi (π)Bonding ◼Pi bonds form after sigma bonds. ◼Sideways overlap of parallel p orbitals
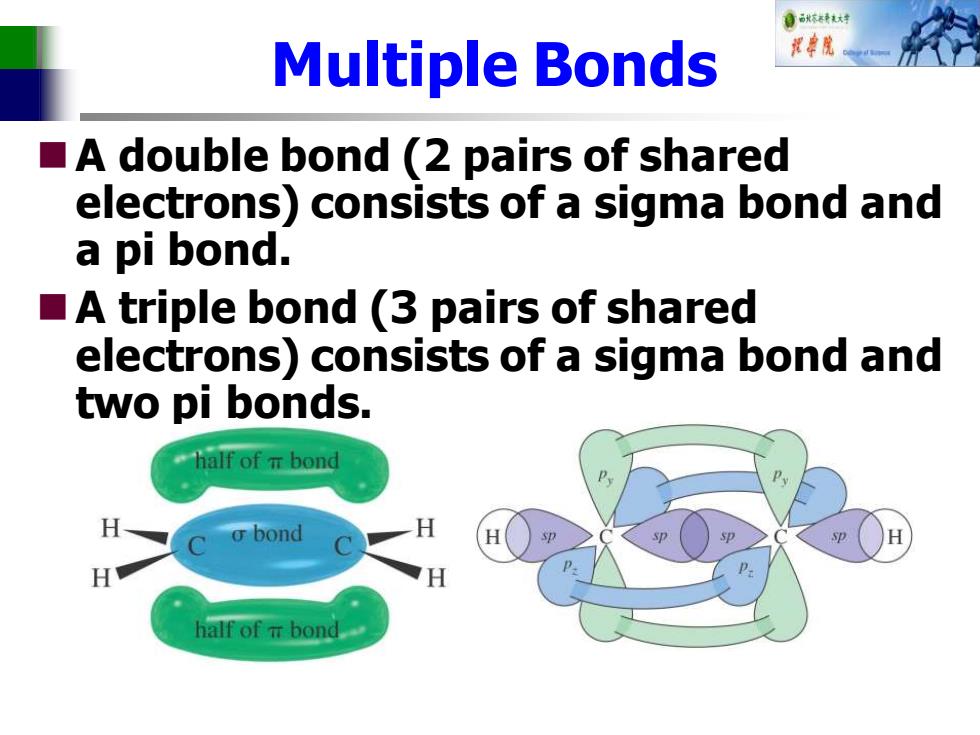
自秋不特大对 Multiple Bonds A double bond (2 pairs of shared electrons)consists of a sigma bond and a pi bond. A triple bond (3 pairs of shared electrons)consists of a sigma bond and two pi bonds. half of m bond o bond H H half of m bond
Multiple Bonds ◼A double bond (2 pairs of shared electrons) consists of a sigma bond and a pi bond. ◼A triple bond (3 pairs of shared electrons) consists of a sigma bond and two pi bonds

SEC 2 Hybridization Molecular Shapes Molecular Shapes ■ Bond angles cannot be explained with simple s and porbitals.Use VSEPR theory. pronounced "vesper" Valence Shell Electron Pair Repulsion Theory 180° 109.5° 109.5° 121 116.6° 109.5° methane,109.5 ethylene,close to 120 acetylene,l80°
SEC 2 Hybridization & Molecular Shapes Molecular Shapes ◼Bond angles cannot be explained with simple s and p orbitals. Use VSEPR theory. ⚫ Valence Shell Electron Pair Repulsion Theory pronounced "vesper

SEC 2 Hybridization Molecular Shapes ■Hybridized orbitals are lower in energy because electron pairs are farther apart. Hybridization is LCAO Linus Pauling (1901-1994)was Within one atom just ihe firsl person ever to receive io prior to bonding. unshared Nobel prizes.He received the 1954 Nobel prize in chemistry for his contributions to our understanding linear combination of atomic orbitals--- of chemical bonding.He received the LCAO 1962 Nobel peace prize for his efforts on behalf of international control of nuclear weapons testing
SEC 2 Hybridization & Molecular Shapes ◼Hybridized orbitals are lower in energy because electron pairs are farther apart. ◼Hybridization is LCAO within one atom, just prior to bonding. linear combination of atomic orbitals--- LCAO