Sigma(o)Bonding ■ Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p or hybridized orbital overlaps. ■ The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals
Sigma(σ) Bonding ◼Electron density lies between the nuclei. ◼A bond may be formed by s-s, p-p, s-p, or hybridized orbital overlaps. ◼The bonding MO is lower in energy than the original atomic orbitals. ◼The antibonding MO is higher in energy than the atomic orbitals
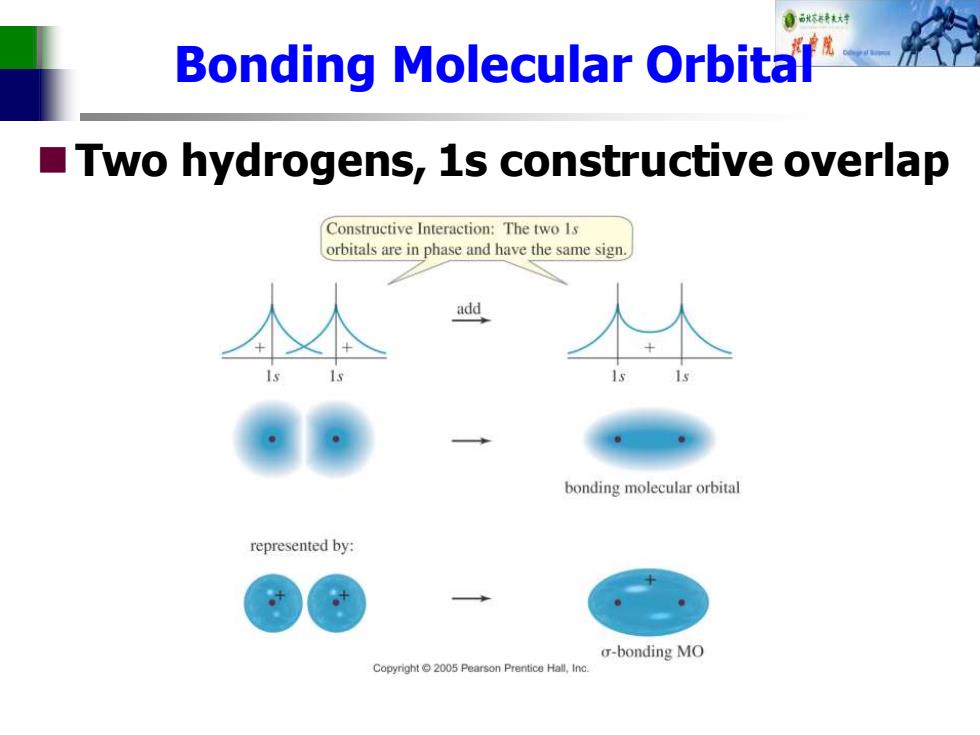
Bonding Molecular Orbital Two hydrogens,1s constructive overlap Constructive Interaction:The two Is orbitals are in phase and have the same sign. add bonding molecular orbital represented by: o-bonding MO Copyright2005 Pearson Prentice Hall,Inc
Bonding Molecular Orbital ◼Two hydrogens, 1s constructive overlap
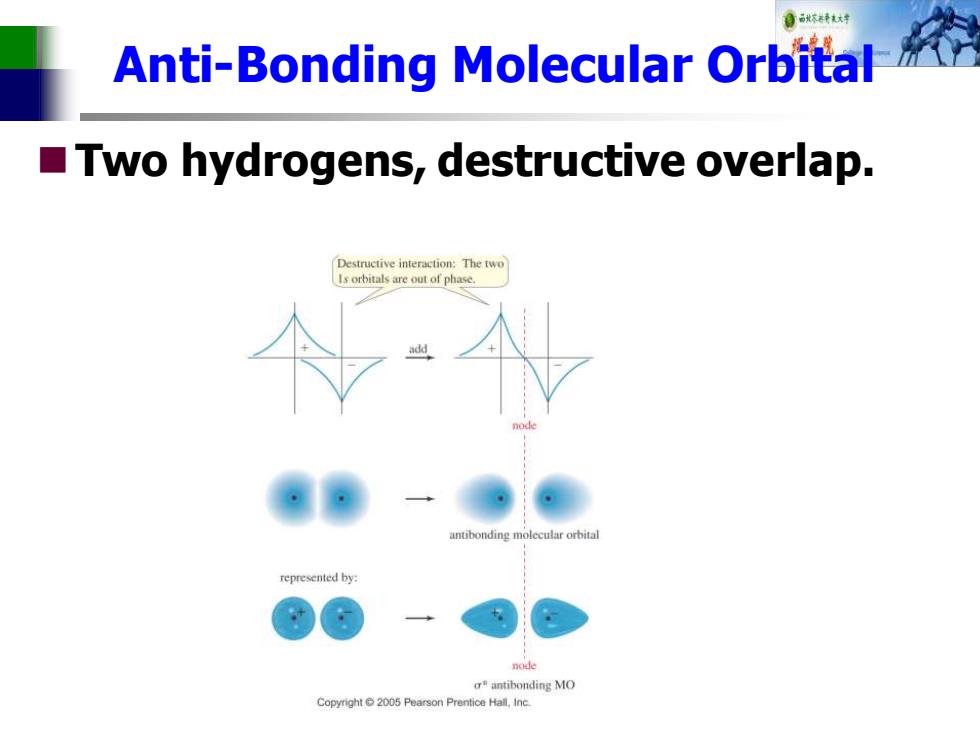
自秋对 Anti-Bonding Molecular Orbital Two hydrogens,destructive overlap. Destructive interaction:The two Is orbitals are out of phase. anubonding molecular orbital represented by: node r产antibonding MO Copyright2005 Pearson Prentice Hall,Inc
Anti-Bonding Molecular Orbital ◼Two hydrogens, destructive overlap
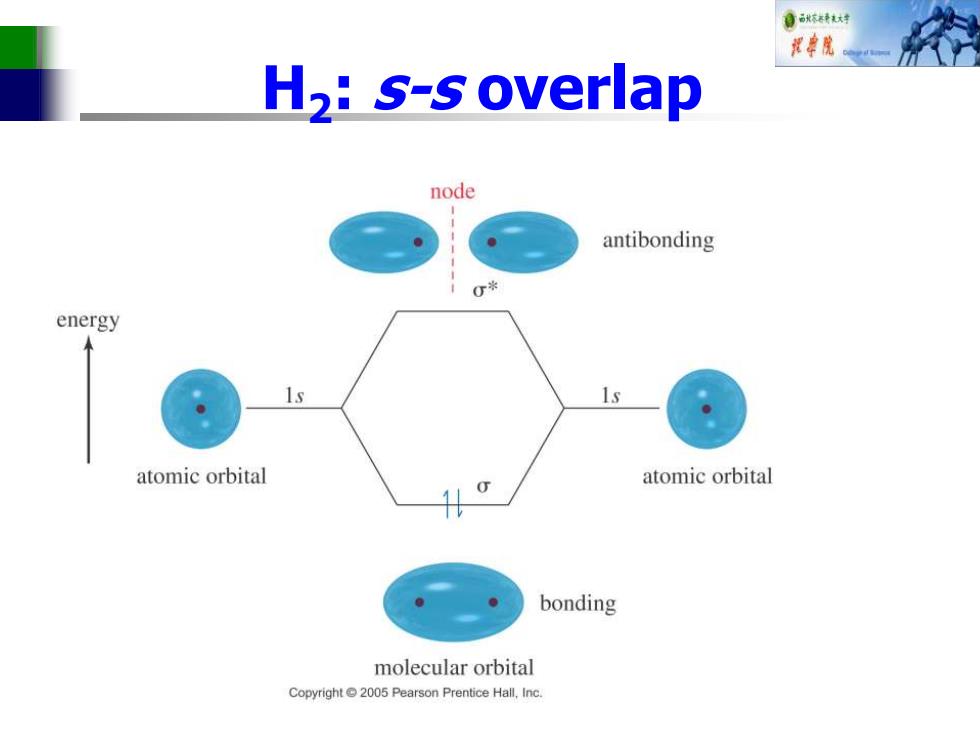
自秋转大材 花单院 H,:s-s overlap node antibonding 0* energy atomic orbital atomic orbital bonding molecular orbital Copyright 2005 Pearson Prentice Hall,Inc
H2 : s-s overlap
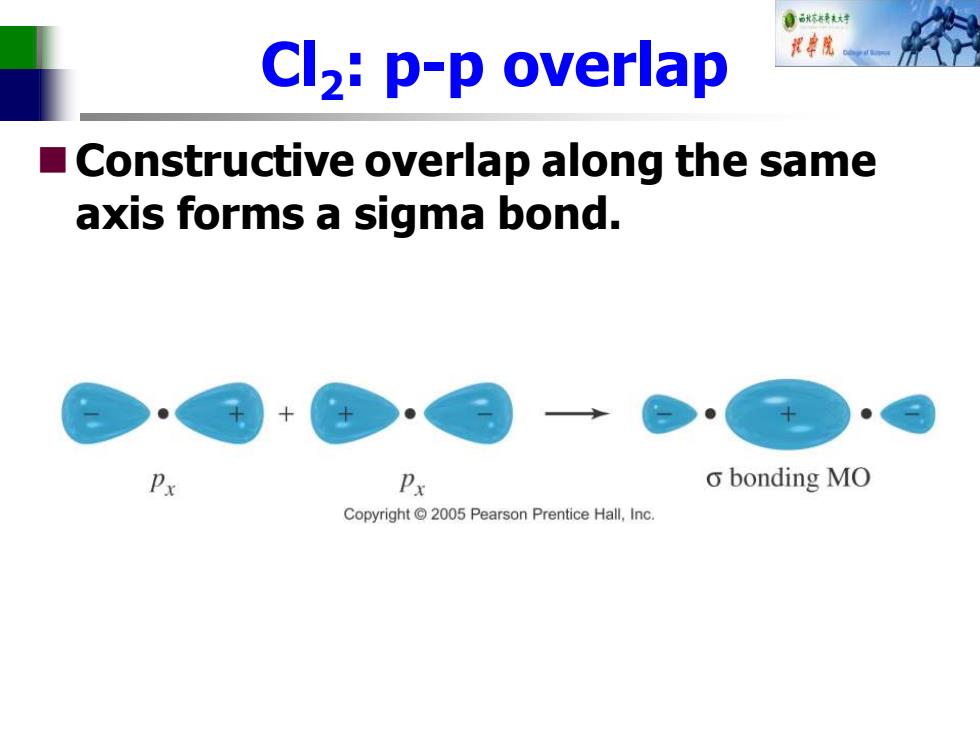
自秋不转大对 Cl2:p-p overlap 中院 Constructive overlap along the same axis forms a sigma bond. Px o bonding MO Copyright 2005 Pearson Prentice Hall,Inc
Cl2 : p-p overlap ◼Constructive overlap along the same axis forms a sigma bond