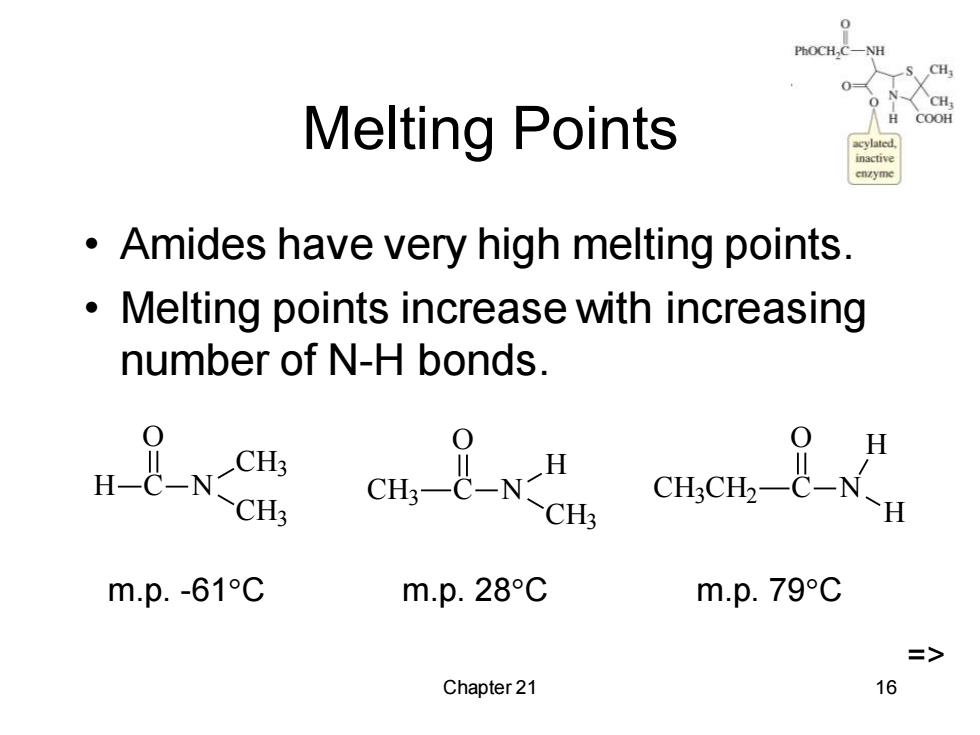
PhOCH,C CH Melting Points COOH Amides have very high melting points. Melting points increase with increasing number of N-H bonds. CH3 m.p.-61C m.p.28C m.p.79C => Chapter 21 16
Chapter 21 16 Melting Points • Amides have very high melting points. • Melting points increase with increasing number of N-H bonds. H C O N CH3 CH3 CH3 C O N H CH3 CH3 CH2 C O N H H m.p. -61C m.p. 28C m.p. 79C =>

H Solubility H COOH Acid chlorides and anhydrides are too inactive reactive to be used with water or alcohol. Esters,3 amides,and nitriles are good polar aprotic solvents. Solvents commonly used in organic reactions: >Ethyl acetate >Dimethylformamide (DMF) >Acetonitrile Chapter 21 17
Chapter 21 17 Solubility • Acid chlorides and anhydrides are too reactive to be used with water or alcohol. • Esters, 3 amides, and nitriles are good polar aprotic solvents. • Solvents commonly used in organic reactions: ➢Ethyl acetate ➢Dimethylformamide (DMF) ➢Acetonitrile =>
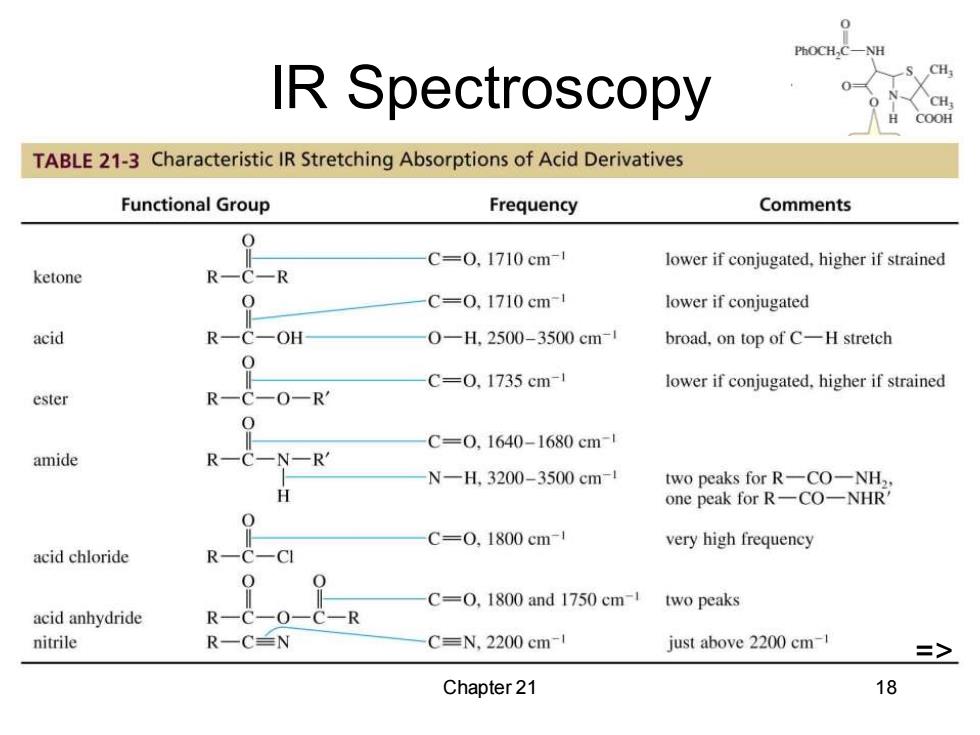
IR Spectroscopy CH COOH TABLE 21-3 Characteristic IR Stretching Absorptions of Acid Derivatives Functional Group Frequency Comments C=0,1710cm lower if conjugated,higher if strained ketone C=0,1710cm- lower if conjugated acid -OH 0-H.2500-3500cm-1 broad,on top of C-H stretch C=0,1735cm- lower if conjugated,higher if strained ester -R C=0,1640-1680cm-1 amide N—RM N-H,3200-3500cm-1 two peaks for R一CO一NH2 one peak for R-CO-NHR' C=0,1800cm- very high frequency acid chloride C=O,1800 and 1750 cm-1 two peaks acid anhydride nitrile R一CN C=N,2200 cm-1 just above 2200 cm-1 => Chapter 21 18
Chapter 21 18 IR Spectroscopy => =>
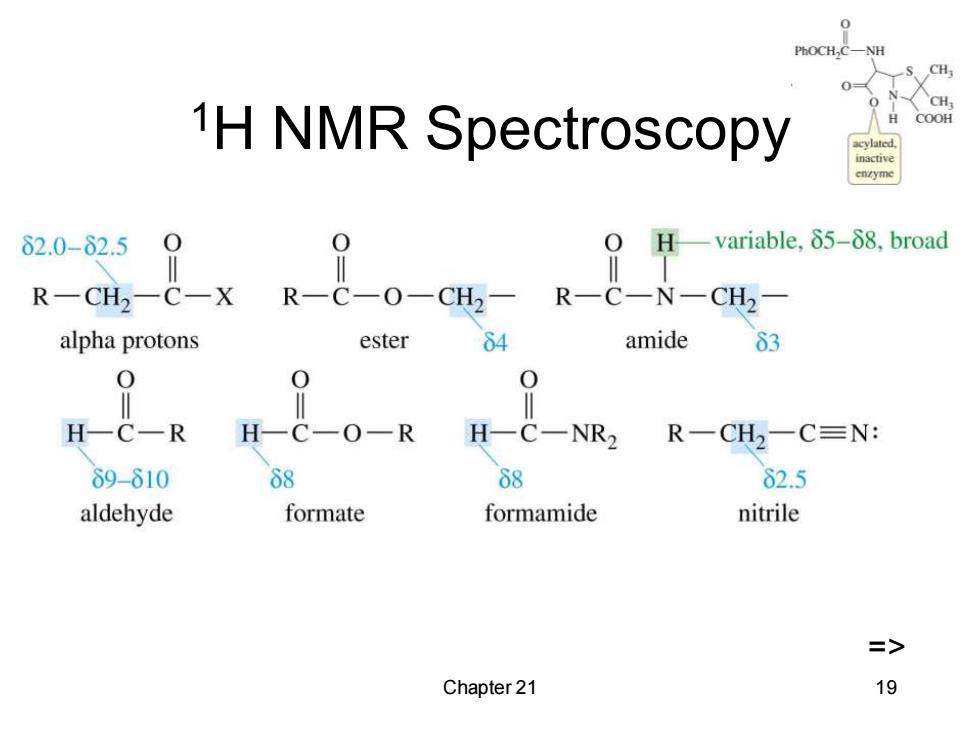
PhOCH,C CH H 1H NMR Spectroscopy COOH cylated inactive 62.0-62.5 0 0 Hvariable,δ5-δ8,broad R-CH2-C-X R-C-O-CH2- R一C-N-CH2 alpha protons ester 64 amide 63 0 H-C一R H-C一O一R H-C-NR2 R一CH2一C≡N: δ9-610 68 68 82.5 aldehyde formate formamide nitrile => Chapter 21 19
Chapter 21 19 1H NMR Spectroscopy =>
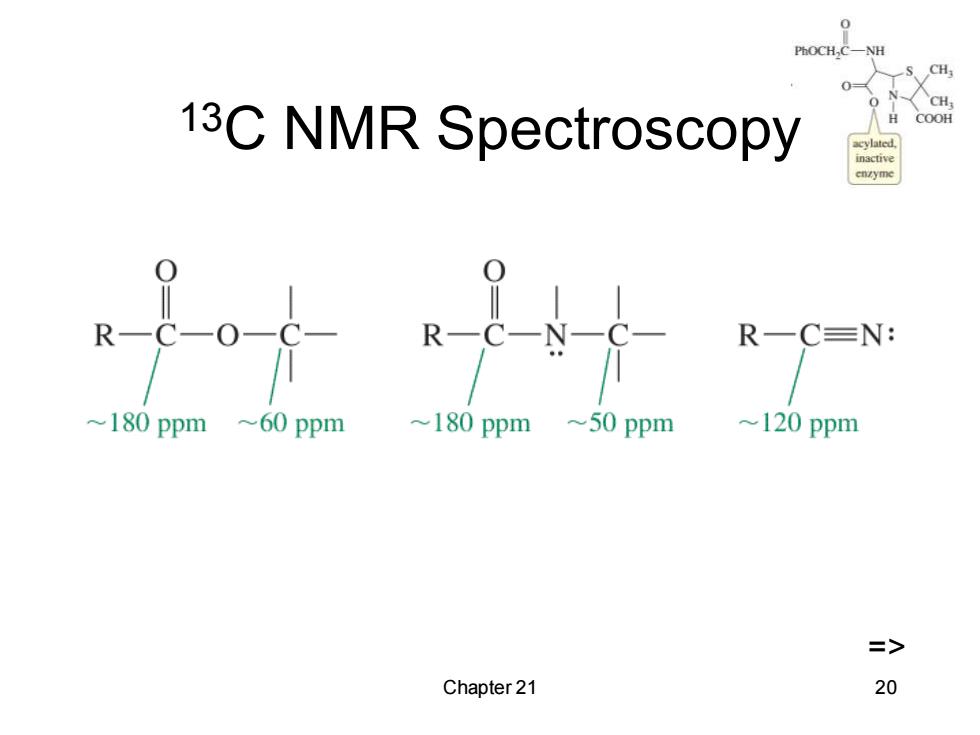
PhOCH, CH CH 13C NMR Spectroscopy COOH inactive R一C=N: 180 ppm ~60 ppm ~180 ppm ~50 ppm ~120 ppm => Chapter 21 20
Chapter 21 20 13C NMR Spectroscopy =>