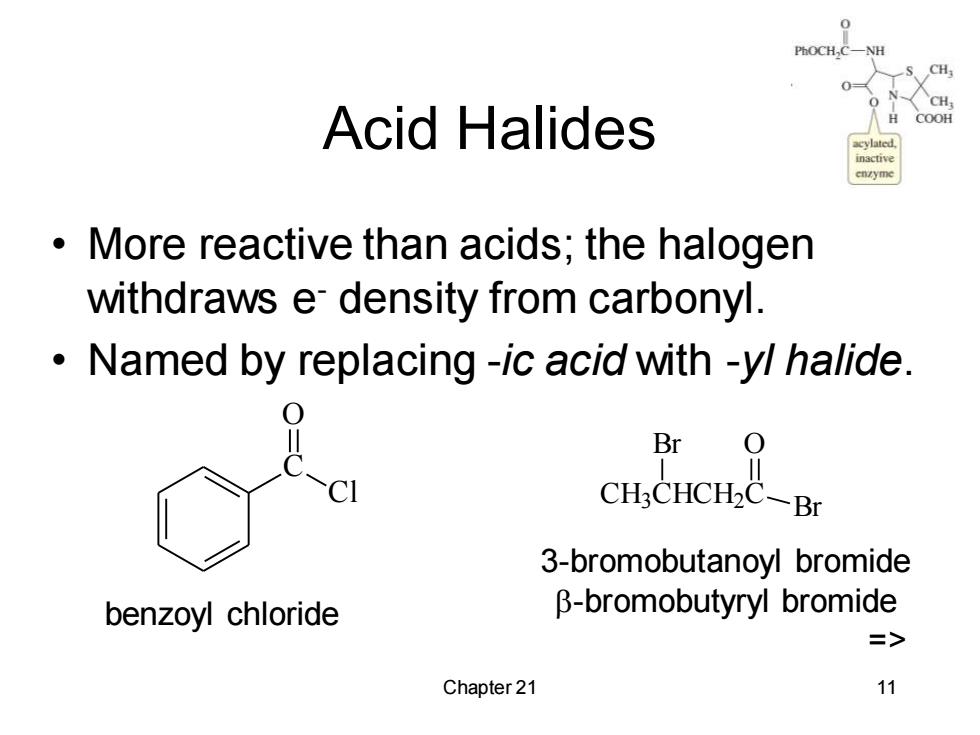
Acid Halides COOH More reactive than acids;the halogen withdraws e density from carbonyl. Named by replacing -ic acid with -yl halide. Br 0 CH3CHCH2C-Br 3-bromobutanoyl bromide benzoyl chloride B-bromobutyryl bromide => Chapter 21 11
Chapter 21 11 Acid Halides • More reactive than acids; the halogen withdraws e- density from carbonyl. • Named by replacing -ic acid with -yl halide. C O Cl CH3 CHCH2 C Br O Br benzoyl chloride 3-bromobutanoyl bromide -bromobutyryl bromide =>
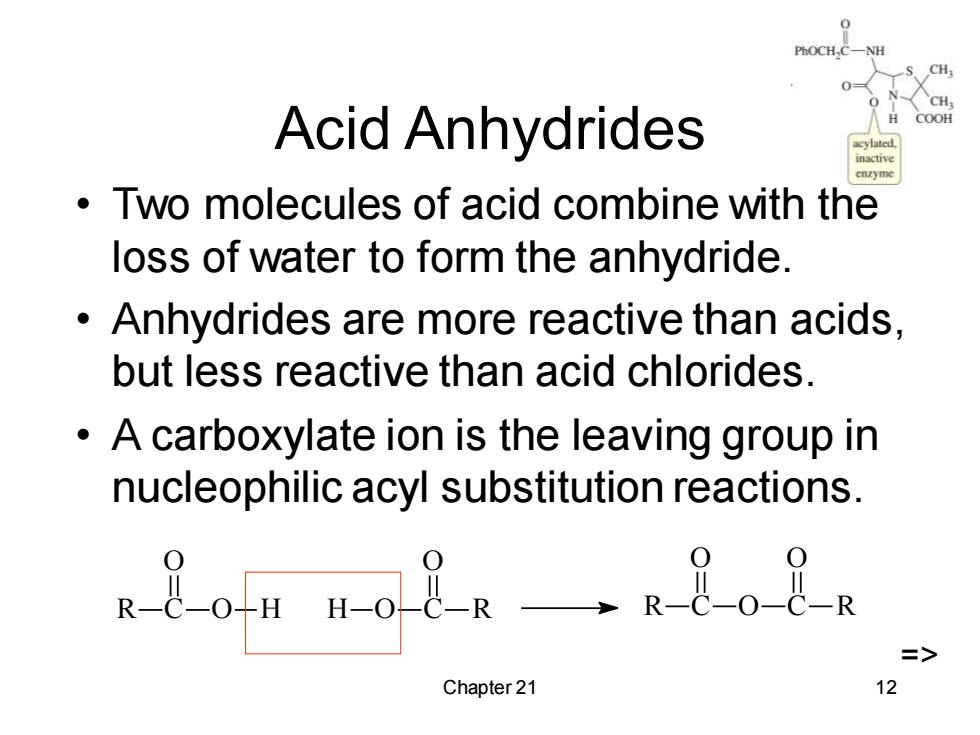
H Acid Anhydrides COOH Two molecules of acid combine with the loss of water to form the anhydride. Anhydrides are more reactive than acids. but less reactive than acid chlorides. A carboxylate ion is the leaving group in nucleophilic acyl substitution reactions. 8on no8R-RBo2a => Chapter 21 12
Chapter 21 12 Acid Anhydrides • Two molecules of acid combine with the loss of water to form the anhydride. • Anhydrides are more reactive than acids, but less reactive than acid chlorides. • A carboxylate ion is the leaving group in nucleophilic acyl substitution reactions. R C O O H C R O H O R C O O C O R =>
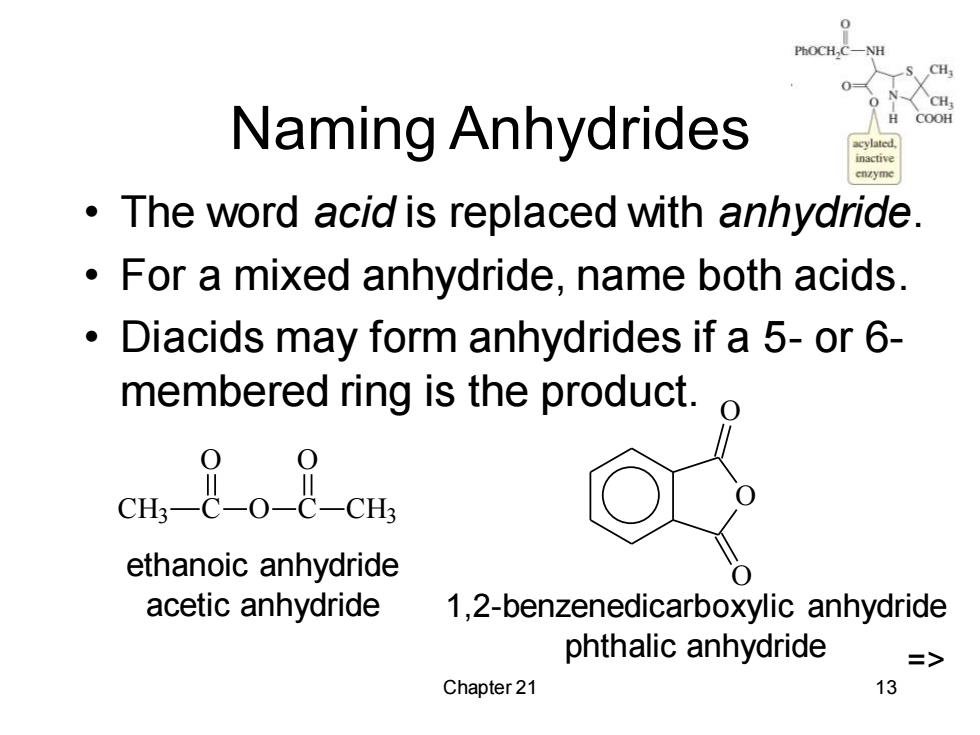
Naming Anhydrides COOH inactive The word acid is replaced with anhydride. For a mixed anhydride,name both acids. Diacids may form anhydrides if a 5-or 6- membered ring is the product. ethanoic anhydride acetic anhydride 1,2-benzenedicarboxylic anhydride phthalic anhydride => Chapter 21 13
Chapter 21 13 Naming Anhydrides • The word acid is replaced with anhydride. • For a mixed anhydride, name both acids. • Diacids may form anhydrides if a 5- or 6- membered ring is the product. CH3 C O O C O CH3 ethanoic anhydride acetic anhydride O O O 1,2-benzenedicarboxylic anhydride phthalic anhydride =>
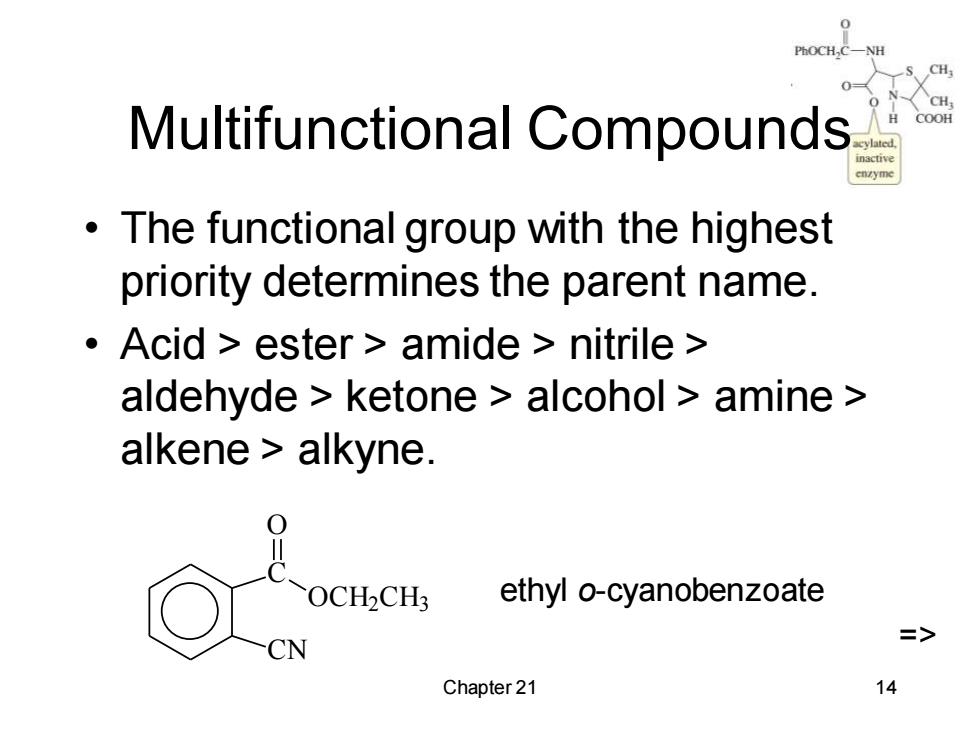
PhOCH, Multifunctional Compounds COOH The functional group with the highest priority determines the parent name. Acid ester amide nitrile aldehyde ketone alcohol amine alkene alkyne. OCH2CH3 ethyl o-cyanobenzoate CN Chapter 21 14
Chapter 21 14 Multifunctional Compounds • The functional group with the highest priority determines the parent name. • Acid > ester > amide > nitrile > aldehyde > ketone > alcohol > amine > alkene > alkyne. C CN O OCH2CH3 ethyl o-cyanobenzoate =>
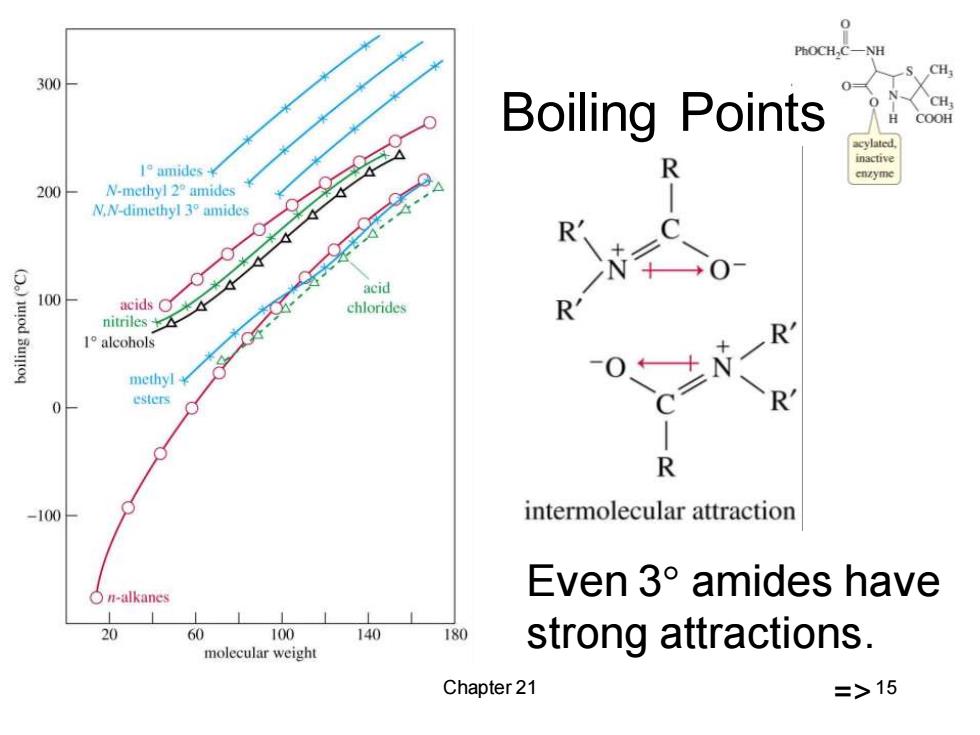
PhOCH,C- CH, 300 Boiling Points H COOH inactive 1°amides¥ 200 N-methyl2°amides N.N-dimethyl 3 amides ● acid 100 acids chlorides nitriles 1 alcohols R nethyl¥ 0 esters R -100 intermolecular attraction n-alkanes Even3°amides have 20 60 100 140 180 strong attractions. molecular weight Chapter 21 =>15
Chapter 21 15 Boiling Points Even 3 amides have strong attractions. =>