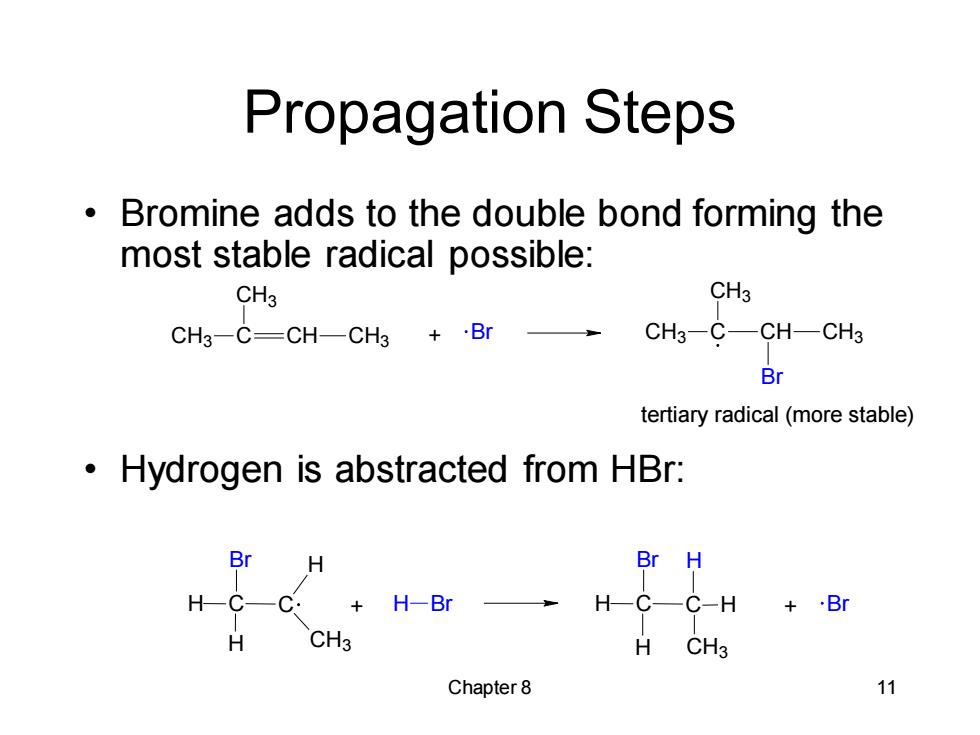
Propagation Steps Bromine adds to the double bond forming the most stable radical possible: CH3 CH3 CH3-C=CH-CH3 +Br CH3-C-CH-CH3 Br tertiary radical(more stable) Hydrogen is abstracted from HBr: Br H Br H H-C-C: H-Br H-C—C-H+B H CH3 H CH3 Chapter 8 11
Chapter 8 11 Propagation Steps • Bromine adds to the double bond forming the most stable radical possible: • Hydrogen is abstracted from HBr: CH + Br 3 C CH3 CH CH3 CH3 C CH3 CH CH3 Br tertiary radical (more stable) H C C H H CH3 Br + H Br H C C H H CH3 Br H + Br
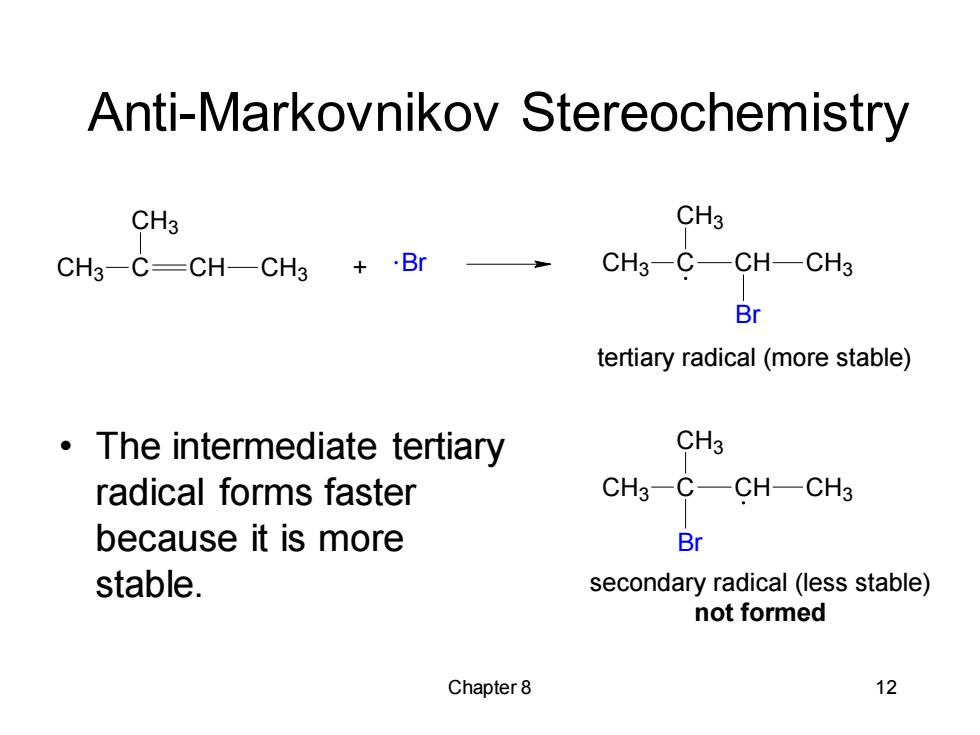
Anti-Markovnikov Stereochemistry CH3 CH3 CH3-C=CH-CH3+·Br CH3-C—CHCH3 Br tertiary radical(more stable) The intermediate tertiary CH3 radical forms faster CH3-C-CH-CH3 because it is more Br stable. secondary radical (less stable) not formed Chapter8 12
Chapter 8 12 Anti-Markovnikov Stereochemistry • The intermediate tertiary radical forms faster because it is more stable. CH + Br 3 C CH3 CH CH3 CH3 C CH3 CH CH3 Br CH3 C CH3 CH CH3 Br secondary radical (less stable) not formed tertiary radical (more stable)

Hydration of Alkenes Hydration of an alkene OH c=( +H,0 alkene alcohol (Markovnikov orientation) The Markovnikov addition of water to the double bond forms an alcohol. This is the reverse of the dehydration of alcohol. Uses dilute solutions of H2SO4 or H3PO4 to drive equilibrium toward hydration. Chapter8 13
Chapter 8 13 Hydration of Alkenes • The Markovnikov addition of water to the double bond forms an alcohol. • This is the reverse of the dehydration of alcohol. • Uses dilute solutions of H2SO4 or H3PO4 to drive equilibrium toward hydration
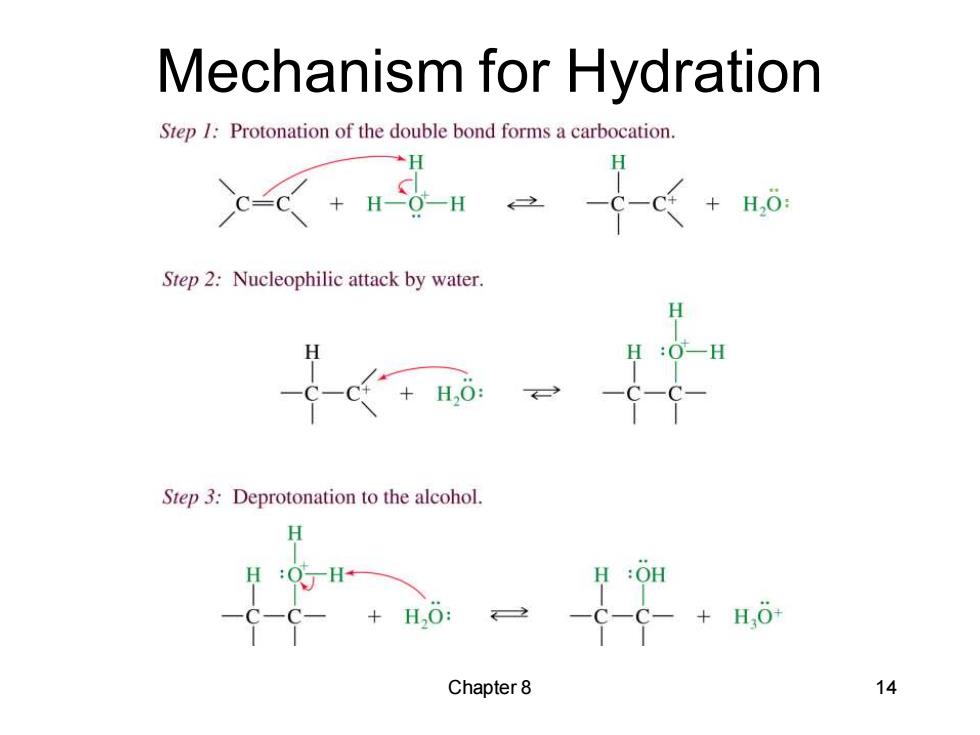
Mechanism for Hydration Step /Protonation of the double bond forms a carbocation. H 《+-H -C-c←+H,0: Step 2:Nucleophilic attack by water. +H,0: Step 3:Deprotonation to the alcohol. H :OH H,O: 2 +HO Chapter8 14
Chapter 8 14 Mechanism for Hydration
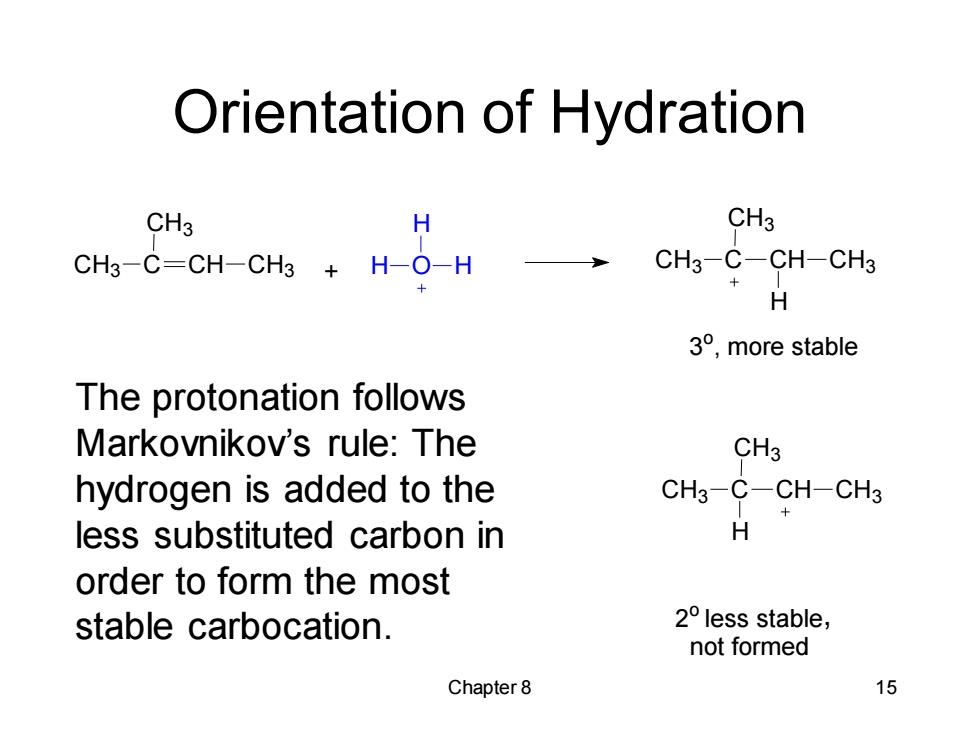
Orientation of Hydration cH CH-CH+H0H CH3 CH3 CH3-C-CH-CH3 H 3°,more stable The protonation follows Markovnikov's rule:The CH3 hydrogen is added to the CH3-C-CH-CH3 less substituted carbon in H order to form the most stable carbocation. 2°less stable, not formed Chapter 8 15
Chapter 8 15 Orientation of Hydration The protonation follows Markovnikov’s rule: The hydrogen is added to the less substituted carbon in order to form the most stable carbocation. CH3 C CH CH3 CH3 + H O H H CH3 C CH CH3 CH3 H 3 o , more stable CH3 C CH CH3 CH3 H 2 o less stable not formed