
3 Alcohols Cannot Be Oxidized Carbon does not have hydrogen,so oxidation is difficult and involves the breakage of a C-C bond. Chromic acid test is for primary and secondary alcohols because tertiary alcohols do not react. Summary of Alcohol Oxidations To Oxidize Product Reagent 2°alcohol ketone chromic acid (or PCC) 1°alcohol aldehyde PCC 1°alcohol carboxylic acid chromic acid Copyright2010 Pearson Prentice Hall,Inc. Chapter 11 11
Chapter 11 11 3° Alcohols Cannot Be Oxidized • Carbon does not have hydrogen, so oxidation is difficult and involves the breakage of a C—C bond. • Chromic acid test is for primary and secondary alcohols because tertiary alcohols do not react

Other Oxidation Reagents ·CuO,300°C(industrial dehydrogenation) Collins reagent:Cr2O3 in pyridine Jones reagent:chromic acid in acetone KMnO4(strong oxidizer) Nitric acid(strong oxidizer) ● Swern oxidation:dimethylsulfoxide,with oxalyl chloride and hindered base, oxidizes 2 alcohols to ketones and 1 alcohols to aldehydes. 12
Chapter 11 12 Other Oxidation Reagents • CuO, 300°C (industrial dehydrogenation) • Collins reagent: Cr2O3 in pyridine • Jones reagent: chromic acid in acetone • KMnO4 (strong oxidizer) • Nitric acid (strong oxidizer) • Swern oxidation: dimethylsulfoxide, with oxalyl chloride and hindered base, oxidizes 2 alcohols to ketones and 1 alcohols to aldehydes
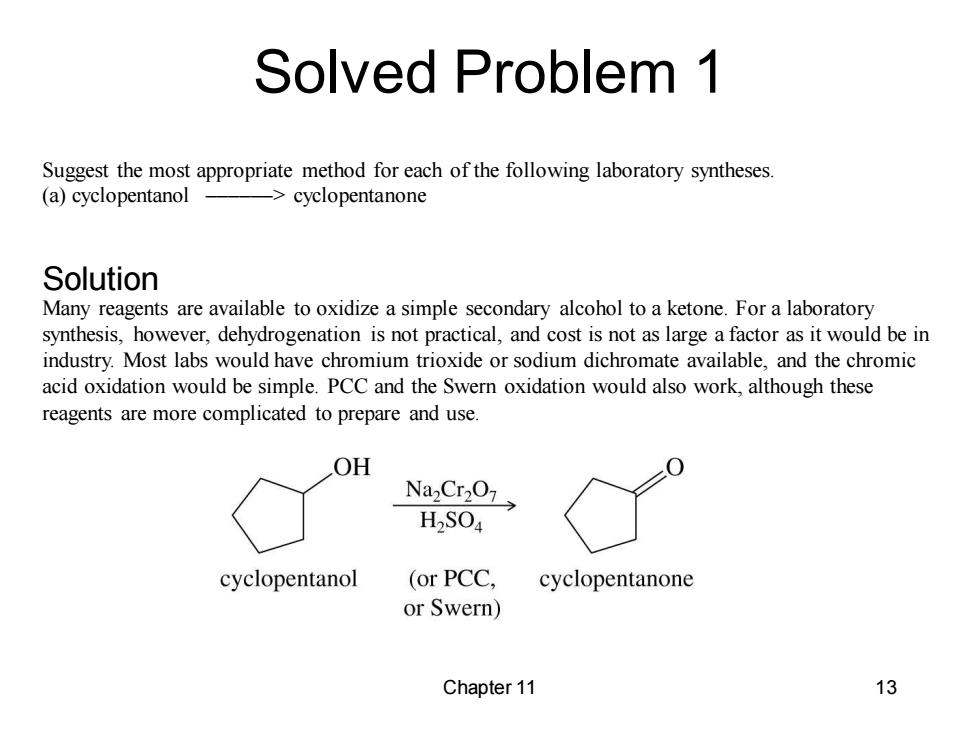
Solved Problem 1 Suggest the most appropriate method for each of the following laboratory syntheses. (a)cyclopentanol ->cyclopentanone Solution Many reagents are available to oxidize a simple secondary alcohol to a ketone.For a laboratory synthesis,however,dehydrogenation is not practical,and cost is not as large a factor as it would be in industry.Most labs would have chromium trioxide or sodium dichromate available,and the chromic acid oxidation would be simple.PCC and the Swern oxidation would also work,although these reagents are more complicated to prepare and use. OH NazCr207> H2SO4 cyclopentanol (or PCC. cyclopentanone or Swern) Chapter 11 13
Chapter 11 13 Suggest the most appropriate method for each of the following laboratory syntheses. (a) cyclopentanol ––––––> cyclopentanone Many reagents are available to oxidize a simple secondary alcohol to a ketone. For a laboratory synthesis, however, dehydrogenation is not practical, and cost is not as large a factor as it would be in industry. Most labs would have chromium trioxide or sodium dichromate available, and the chromic acid oxidation would be simple. PCC and the Swern oxidation would also work, although these reagents are more complicated to prepare and use. Solved Problem 1 Solution
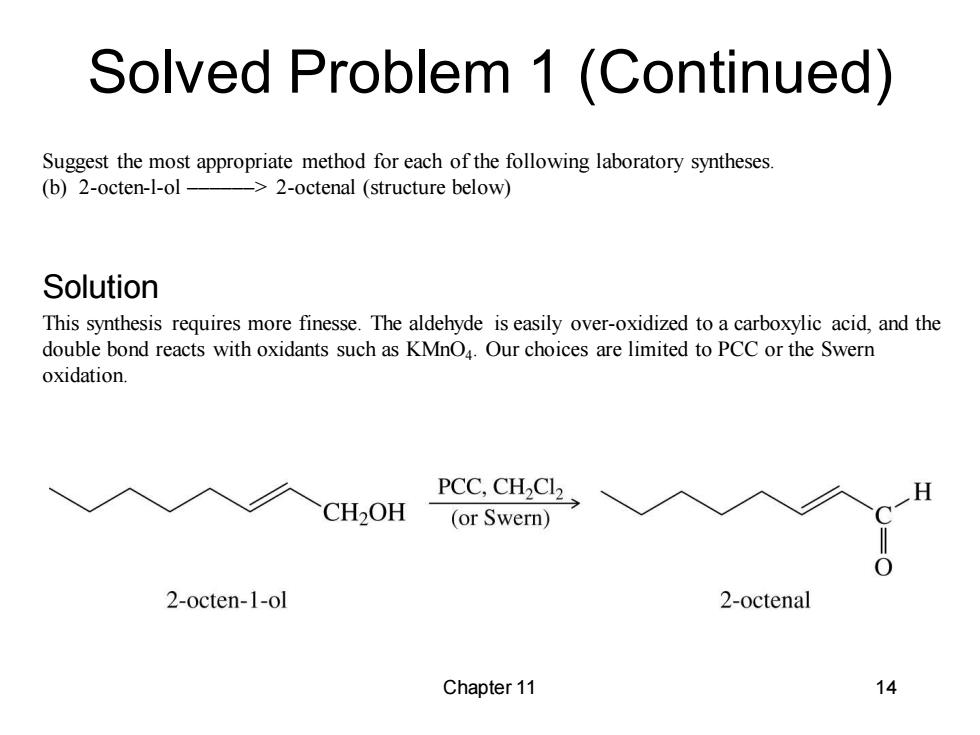
Solved Problem 1 (Continued) Suggest the most appropriate method for each of the following laboratory syntheses. (b)2-octen-1-ol- ->2-octenal (structure below) Solution This synthesis requires more finesse.The aldehyde is easily over-oxidized to a carboxylic acid,and the double bond reacts with oxidants such as KMnO4.Our choices are limited to PCC or the Swern oxidation. PCC,CH2Cl2 CH2OH (or Swern) 2-octen-1-ol 2-octenal Chapter 11 14
Chapter 11 14 Suggest the most appropriate method for each of the following laboratory syntheses. (b) 2-octen-l-ol ––––––> 2-octenal (structure below) This synthesis requires more finesse. The aldehyde is easily over-oxidized to a carboxylic acid, and the double bond reacts with oxidants such as KMnO4 . Our choices are limited to PCC or the Swern oxidation. Solved Problem 1 (Continued) Solution
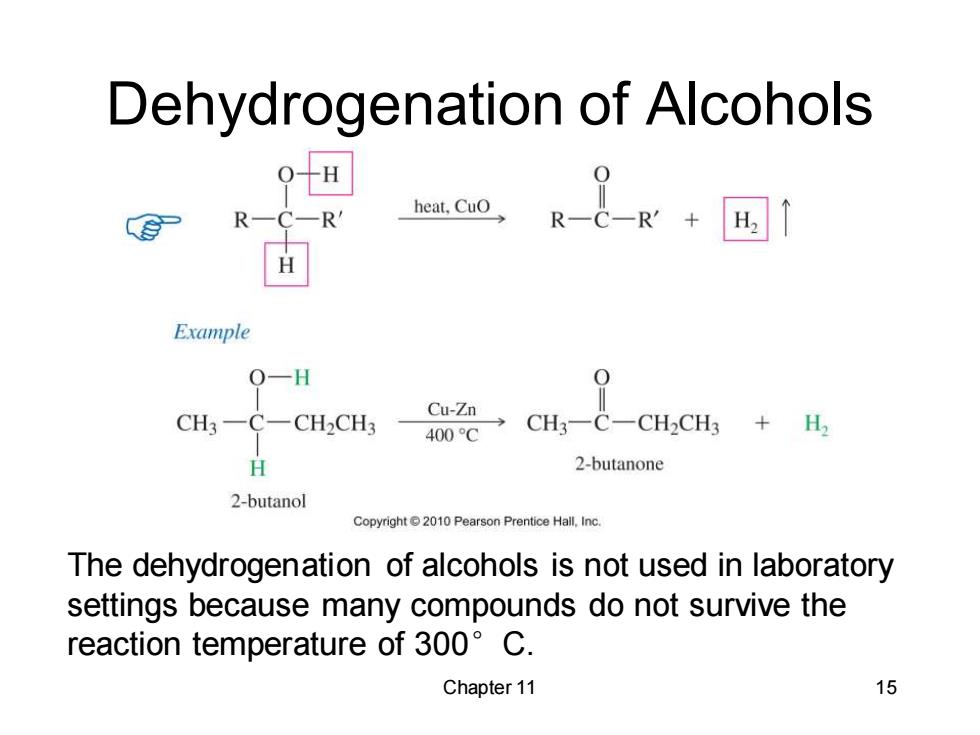
Dehydrogenation of Alcohols Example Q-H CH3-C-CH2CH3 CH3-C-CH2CH3 H. 一400℃ H 2-butanone 2-butanol Copyright2010 Pearson Prentice Hall.Inc. The dehydrogenation of alcohols is not used in laboratory settings because many compounds do not survive the reaction temperature of 300 C. Chapter 11 15
Chapter 11 15 Dehydrogenation of Alcohols The dehydrogenation of alcohols is not used in laboratory settings because many compounds do not survive the reaction temperature of 300°C