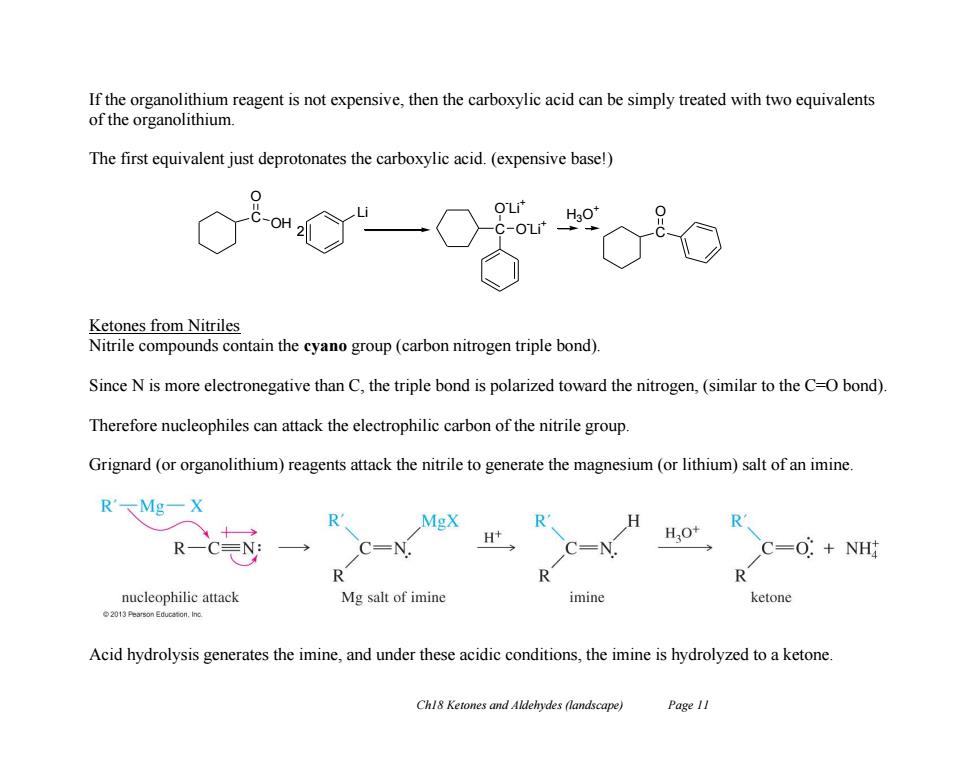
If the organolithium reagent is not expensive,then the carboxylic acid can be simply treated with two equivalents of the organolithium The first equivalent just deprotonates the carboxylic acid.(expensive base!) OH Ketones from Nitriles Nitrile compounds contain the cyano group(carbon nitrogen triple bond). Since N is more electronegative than C,the triple bond is polarized toward the nitrogen,(similar to the C-O bond) Therefore nucleophiles can attack the electrophilic carbon of the nitrile group. Grignard (or organolithium)reagents attack the nitrile to generate the magnesium(or lithium)salt of an imine. R'MgX R MgX R R H+ HO+ RC EN: C=0:NH R R nucleophilic attack Mg salt of imine imine ketone 2013 Pearson Educeton.Ino. Acid hydrolysis generates the imine,and under these acidic conditions,the imine is hydrolyzed to a ketone. Chl8 Ketones and Aldehydes (landscape) Page 11
Ch18 Ketones and Aldehydes (landscape) Page 11 If the organolithium reagent is not expensive, then the carboxylic acid can be simply treated with two equivalents of the organolithium. The first equivalent just deprotonates the carboxylic acid. (expensive base!) Ketones from Nitriles Nitrile compounds contain the cyano group (carbon nitrogen triple bond). Since N is more electronegative than C, the triple bond is polarized toward the nitrogen, (similar to the C=O bond). Therefore nucleophiles can attack the electrophilic carbon of the nitrile group. Grignard (or organolithium) reagents attack the nitrile to generate the magnesium (or lithium) salt of an imine. Acid hydrolysis generates the imine, and under these acidic conditions, the imine is hydrolyzed to a ketone. C OH O Li 2 C O - Li + O - Li + C H O 3O +
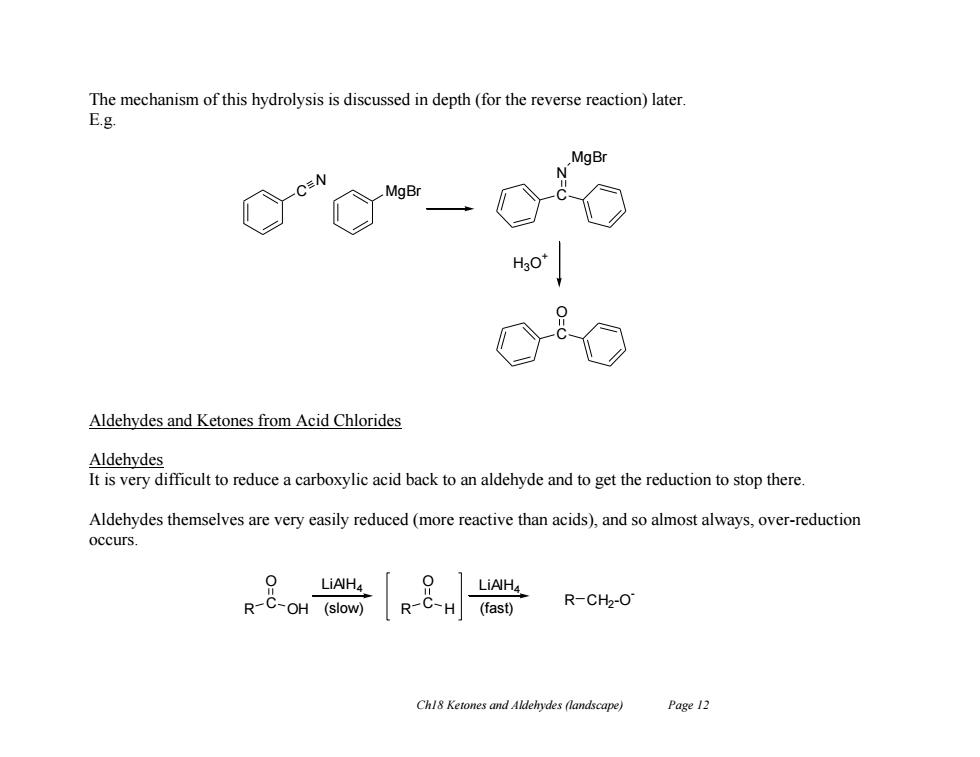
The mechanism of this hydrolysis is discussed in depth(for the reverse reaction)later. E.g. MgBr H3 Aldehydes and Ketones from Acid Chlorides Aldehydes It is very difficult to reduce a carboxylic acid back to an aldehyde and to get the reduction to stop there. Aldehydes themselves are very easily reduced (more reactive than acids),and so almost always,over-reduction occurs. 0 LiAH4 0 LiAH4 R-C-OH (slow) R-C-H(fast) R-CH2-O Chl8 Ketones and Aldehydes (landscape) Page 12
Ch18 Ketones and Aldehydes (landscape) Page 12 The mechanism of this hydrolysis is discussed in depth (for the reverse reaction) later. E.g. Aldehydes and Ketones from Acid Chlorides Aldehydes It is very difficult to reduce a carboxylic acid back to an aldehyde and to get the reduction to stop there. Aldehydes themselves are very easily reduced (more reactive than acids), and so almost always, over-reduction occurs. C N MgBr C N MgBr H3O + C O R C OH O R C H O R CH2 -O - LiAlH4 (slow) LiAlH4 (fast)

However,to circumvent this problem,carboxylic acids can be converted first into a functional group that is easier to reduce than an aldehyde group. The group of choice is an acid chloride The reaction of carboxylic acids with thionyl chloride (SOCl2)generates acid chlorides. 0 0 0 R OH + CI-S-CI R-C-CI+HCI个+SO2↑ acid thionyl chloride acid chloride Although strong reducing agents like LiAlH,still reduce acid chlorides all the way to primary alcohols,milder reducing agents like lithium aluminum tri(butoxy)hydride can selectively reduce acid chlorides to aldehydes. O LiAIH(O-/-Bu) R -C1 R一C一H lithium tri-fert-butoxyaluminum hydride acid chloride aldehyde Example CH CH3 CH, s0C1, CH,CHCH,-C-OH CH,CHCH,一C-CI Li产AHO-+Bu CH,CHCH,一C-H isovaleric acid isovaleroyl chloride isovaleraldehyde(65%) 2013 Pearson Educsticn.Ic. Chl8 Ketones and Aldehydes (landscape) Page 13
Ch18 Ketones and Aldehydes (landscape) Page 13 However, to circumvent this problem, carboxylic acids can be converted first into a functional group that is easier to reduce than an aldehyde group. The group of choice is an acid chloride. The reaction of carboxylic acids with thionyl chloride (SOCl2) generates acid chlorides. Although strong reducing agents like LiAlH4 still reduce acid chlorides all the way to primary alcohols, milder reducing agents like lithium aluminum tri(t butoxy)hydride can selectively reduce acid chlorides to aldehydes
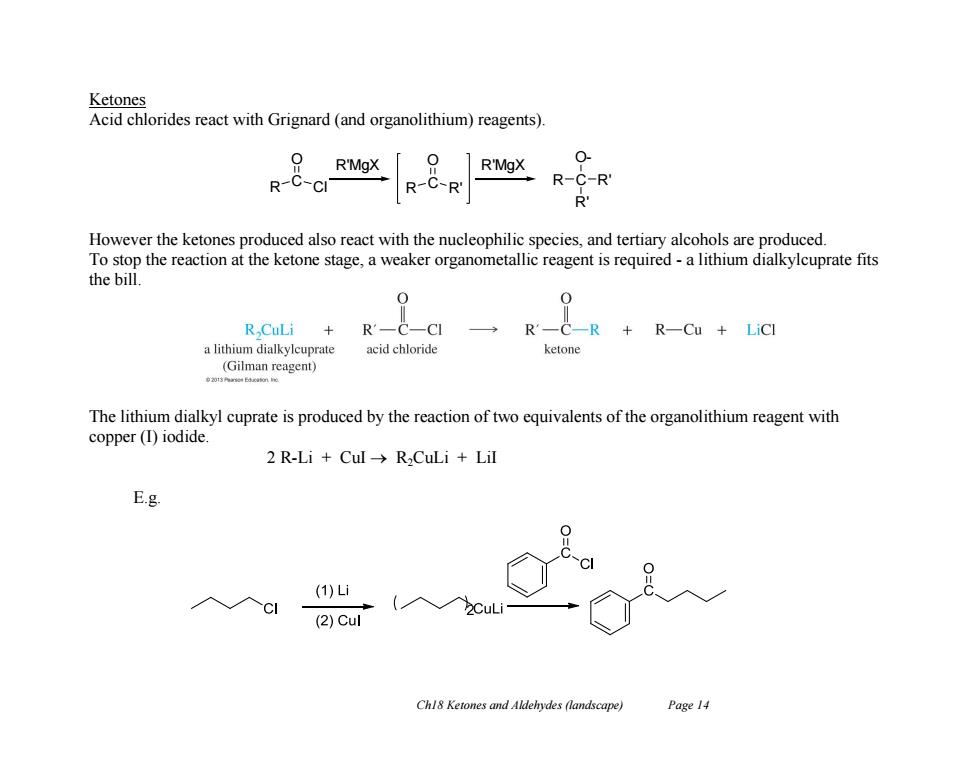
Ketones Acid chlorides react with Grignard (and organolithium)reagents). 0 R'MgX R'MgX R-C-CI R-C-R R-C-R' R However the ketones produced also react with the nucleophilic species,and tertiary alcohols are produced. To stop the reaction at the ketone stage,a weaker organometallic reagent is required-a lithium dialkylcuprate fits the bill. R CuLi R一C R-Cu LiCl a lithium dialkylcuprate acid chloride ketone (Gilman reagent) 河时neb的anm The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper(T)iodide 2 R-Li Cul->R,CuLi Lil E.g (1)i CI (2)Cul Ch18 Ketones and Aldehydes (landscape) Page 14
Ch18 Ketones and Aldehydes (landscape) Page 14 Ketones Acid chlorides react with Grignard (and organolithium) reagents). However the ketones produced also react with the nucleophilic species, and tertiary alcohols are produced. To stop the reaction at the ketone stage, a weaker organometallic reagent is required - a lithium dialkylcuprate fits the bill. The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. 2 R-Li + CuI R2CuLi + LiI E.g. R C Cl O R C R' R'MgX O R'MgX R C O- R' R
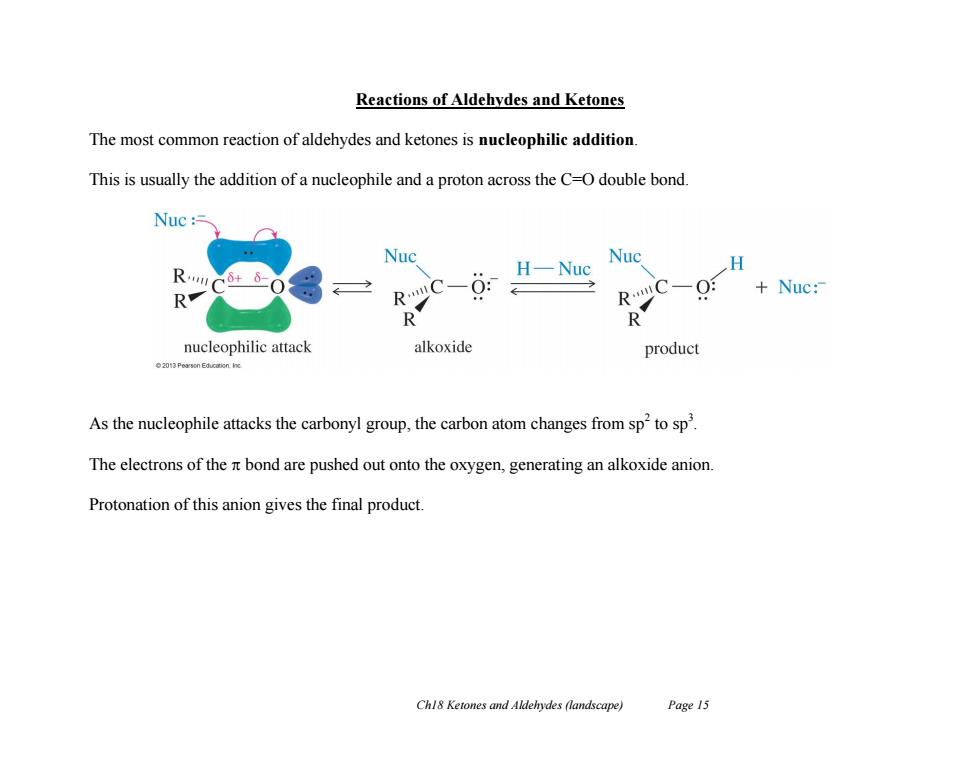
Reactions of Aldehydes and Ketones The most common reaction of aldehydes and ketones is nucleophilic addition This is usually the addition of a nucleophile and a proton across the C-O double bond. Nuc Nuc Nuc R H-Nuc H Nuc: R RC-0: RC-0: R nucleophilic attack alkoxide product ●2013 Peron Eduation ic As the nucleophile attacks the carbonyl group,the carbon atom changes from sp2to sp. The electrons of the nt bond are pushed out onto the oxygen,generating an alkoxide anion Protonation of this anion gives the final product Chl8 Ketones and Aldehydes (landscape) Page 15
Ch18 Ketones and Aldehydes (landscape) Page 15 Reactions of Aldehydes and Ketones The most common reaction of aldehydes and ketones is nucleophilic addition. This is usually the addition of a nucleophile and a proton across the C=O double bond. As the nucleophile attacks the carbonyl group, the carbon atom changes from sp2 to sp3 . The electrons of the bond are pushed out onto the oxygen, generating an alkoxide anion. Protonation of this anion gives the final product