
Acidity R-0-H+H,ǒ:←→ R-0 +H,0*K1016 pK.=16 alcohol alkoxide R-C-0-H+H,0:→ R-C +HO+ pK=5 (Kn≡10-5 acid O: carboxylate R一O- H3O+ R-OH +H2O R 00 HO+ K8Jau3 R一COOH+HO stabilization of carboxylate => Chapter 20 11
Chapter 20 11 Acidity =>
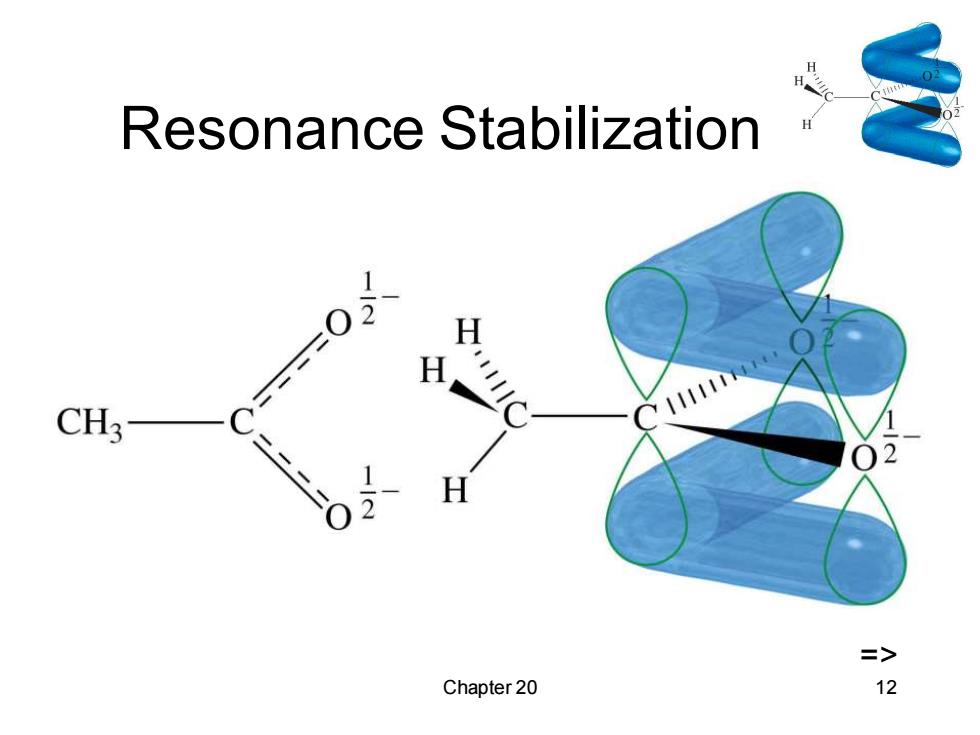
Resonance Stabilization H H CH3 H => Chapter 20 12
Chapter 20 12 Resonance Stabilization =>
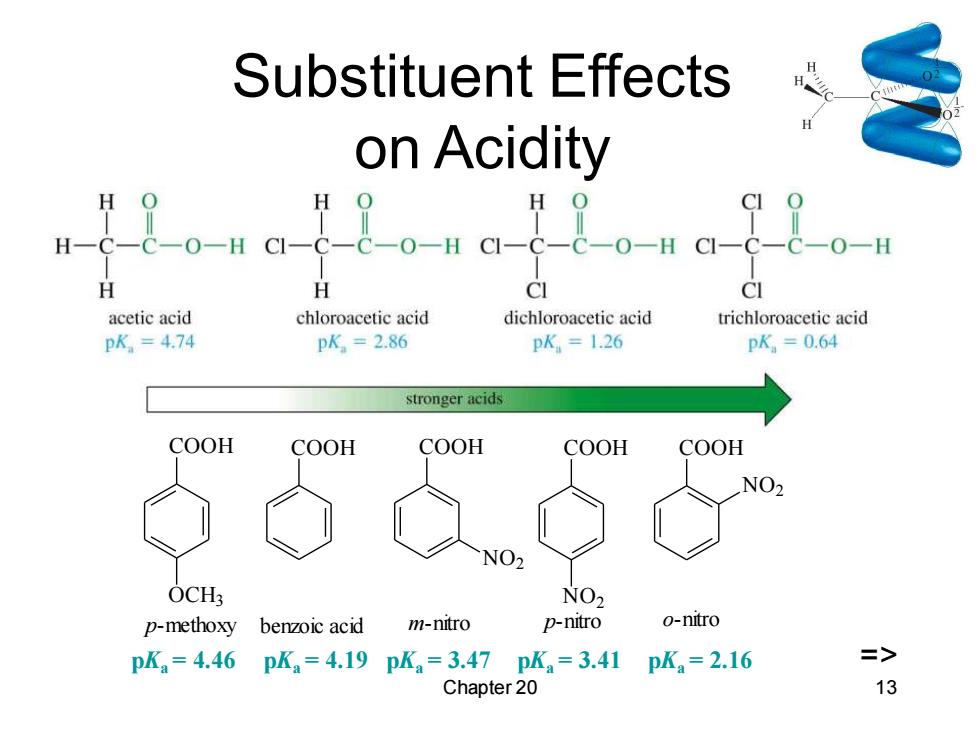
Substituent Effects on Acidity 99 H H CI CI acetic acid chloroacetic acid dichloroacetic acid trichloroacetic acid pK,=474 pK.=2.86 pK,=1.26 pK,=0.64 stronger acids COOH COOH COOH COOH COOH NO2 NO2 OCH: NO2 p-methoxy benzoic acid m-nitro P-nitro o-nitro pK=4.46pK=4.19pKa=3.47pK=3.41 pKa=2.16 => Chapter 20 13
Chapter 20 13 Substituent Effects on Acidity COOH OCH3 COOH COOH NO2 COOH NO2 COOH NO2 p-methoxy benzoic acid m-nitro p-nitro o-nitro pKa = 4.46 pKa = 4.19 pKa = 3.47 pKa = 3.41 pKa = 2.16 =>