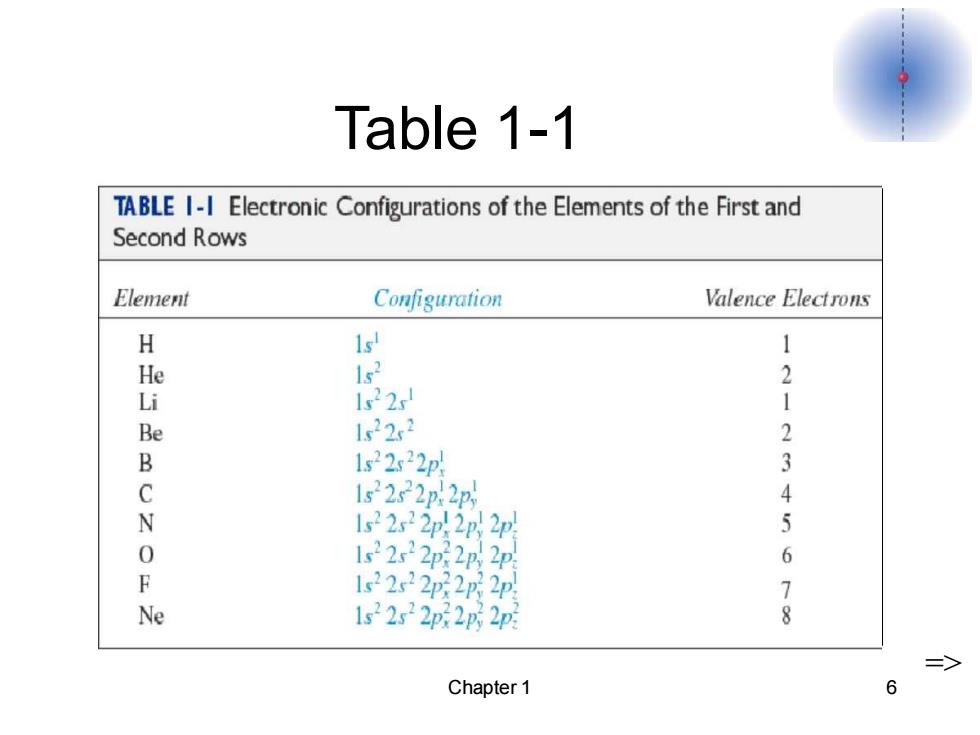
Table 1-1 TABLE I-I Electronic Configurations of the Elements of the First and Second Rows Element Configuration Valence Electrons H 1 1 He 1s2 2 Li 1s22 1 Be 1w22x2 2 B 1s22s22p 3 C 1s2222p2 4 N 122p2p2p 5 0 1s22x22p22n2p 6 F 1s22s22p222p 7 Ne 1s2s2p:2p2p Chapter 1 6
Chapter 1 6 Table 1-1 =>
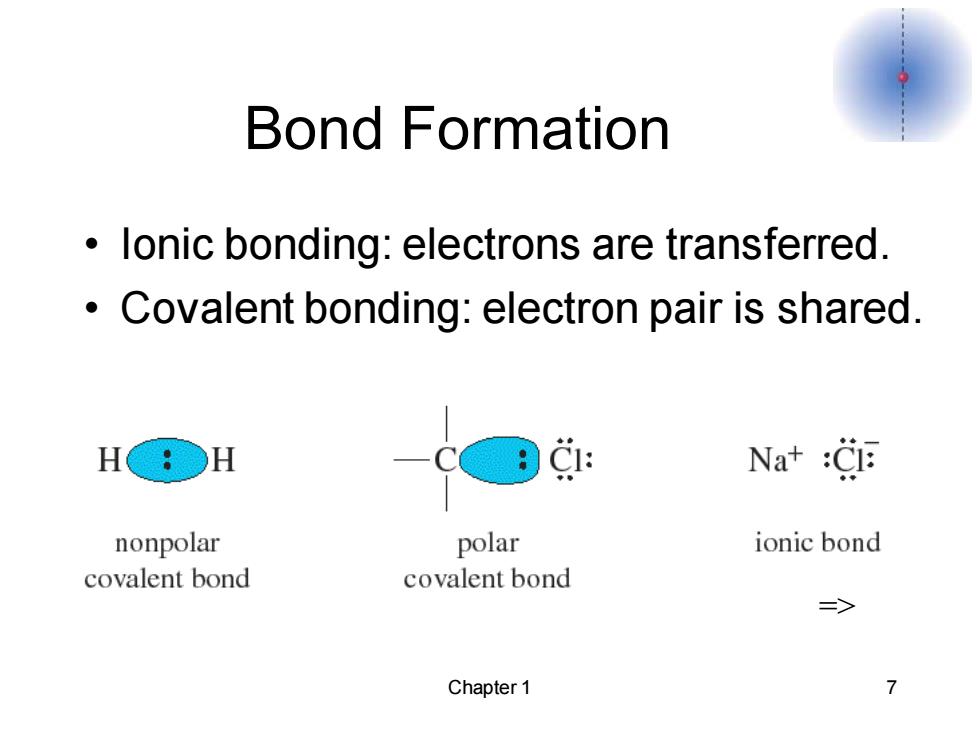
Bond Formation lonic bonding:electrons are transferred. Covalent bonding:electron pair is shared. H○H CE Nat :Cl nonpolar polar ionic bond covalent bond covalent bond => Chapter 1 7
Chapter 1 7 Bond Formation • Ionic bonding: electrons are transferred. • Covalent bonding: electron pair is shared. =>
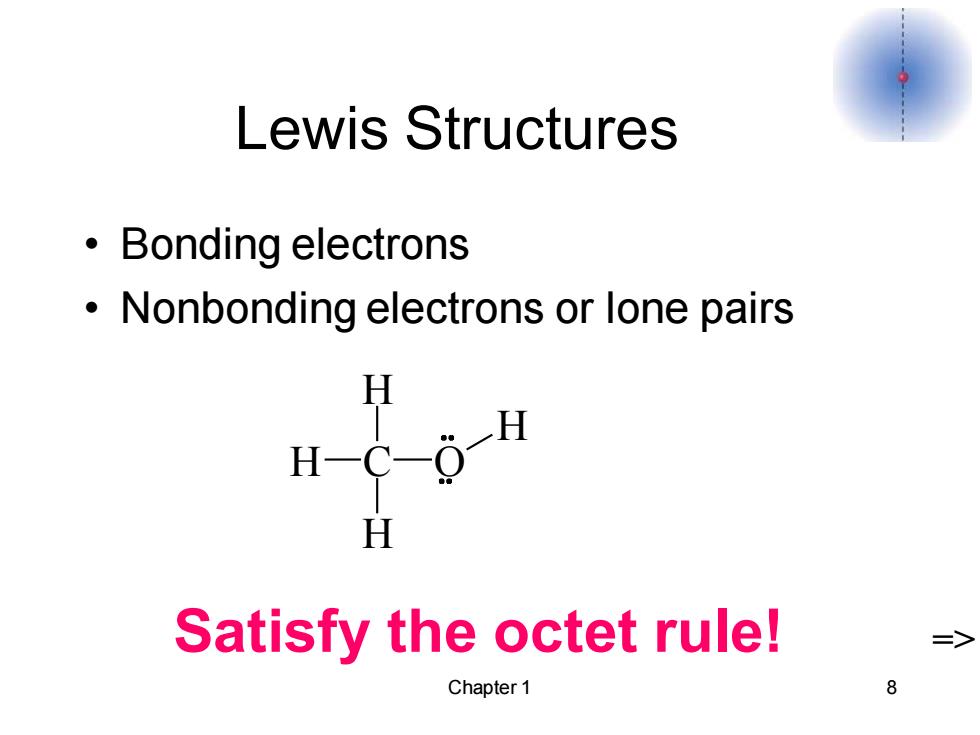
Lewis Structures ·Bonding electrons Nonbonding electrons or lone pairs H H-C-0-H H Satisfy the octet rule! => Chapter 1 8
Chapter 1 8 Lewis Structures • Bonding electrons • Nonbonding electrons or lone pairs Satisfy the octet rule! => C H H H O H
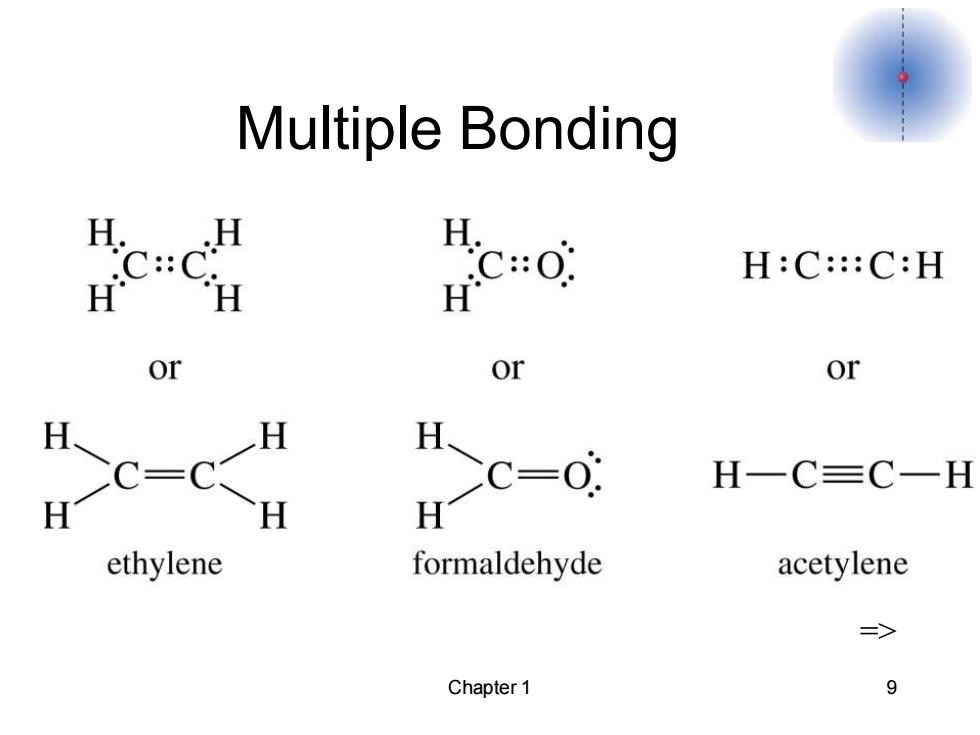
Multiple Bonding he心 H:C:::C:H or or CC- H-c-; H一C三C一H ethylene formaldehyde acetylene > Chapter 1 9
Chapter 1 9 Multiple Bonding =>
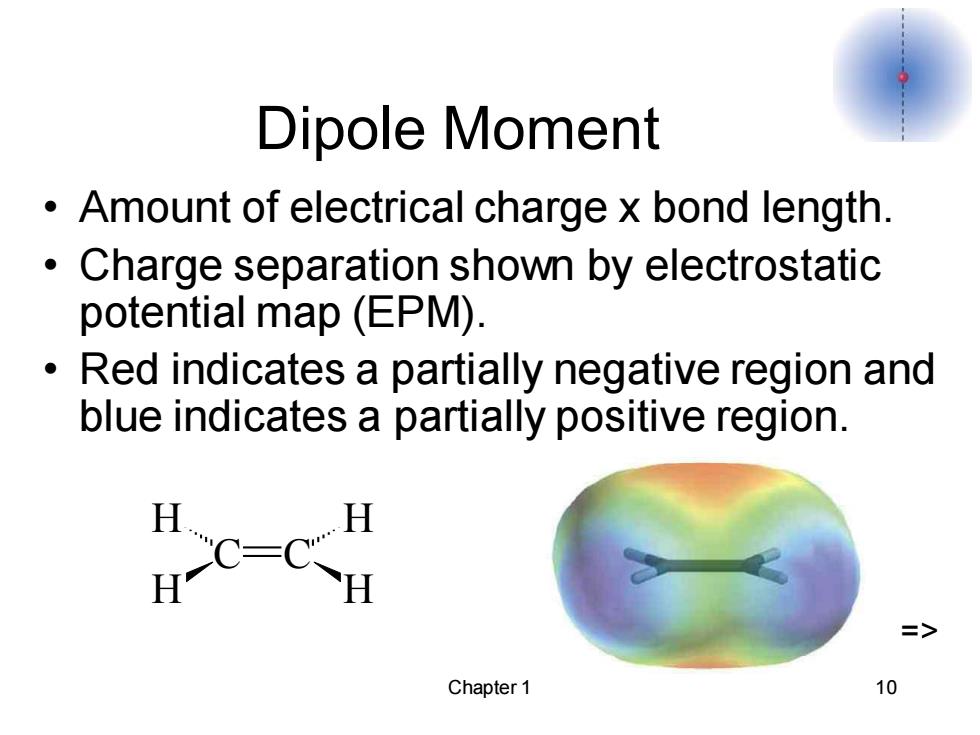
Dipole Moment Amount of electrical charge x bond length. Charge separation shown by electrostatic potential map(EPM). Red indicates a partially negative region and blue indicates a partially positive region. HC-CH => Chapter 1 10
Chapter 1 10 Dipole Moment • Amount of electrical charge x bond length. • Charge separation shown by electrostatic potential map (EPM). • Red indicates a partially negative region and blue indicates a partially positive region. C C H H H H =>