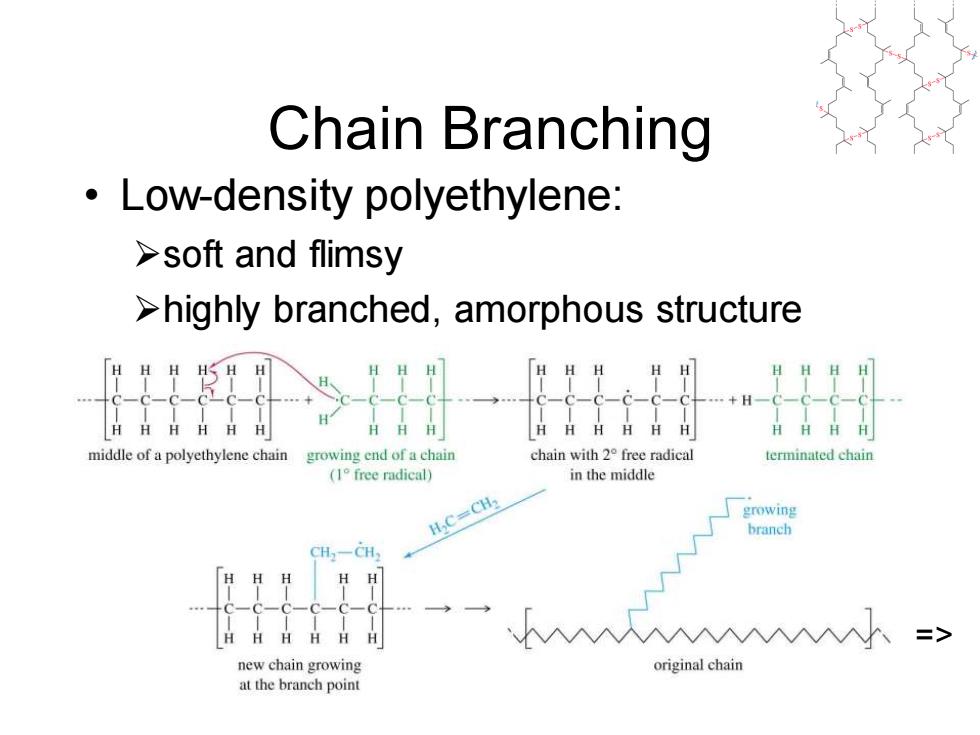
Chain Branching Low-density polyethylene: >soft and flimsy >highly branched,amorphous structure middle of a polyethylene chain growing end of a chain chain with 2 free radical terminated chain (1°free radical) in the middle H2C=CH2 growing branch CH-CH HHH H HHHHH 、=> new chain growing original chain at the branch point
Chapter 26 6 Chain Branching • Low-density polyethylene: ➢soft and flimsy ➢highly branched, amorphous structure =>
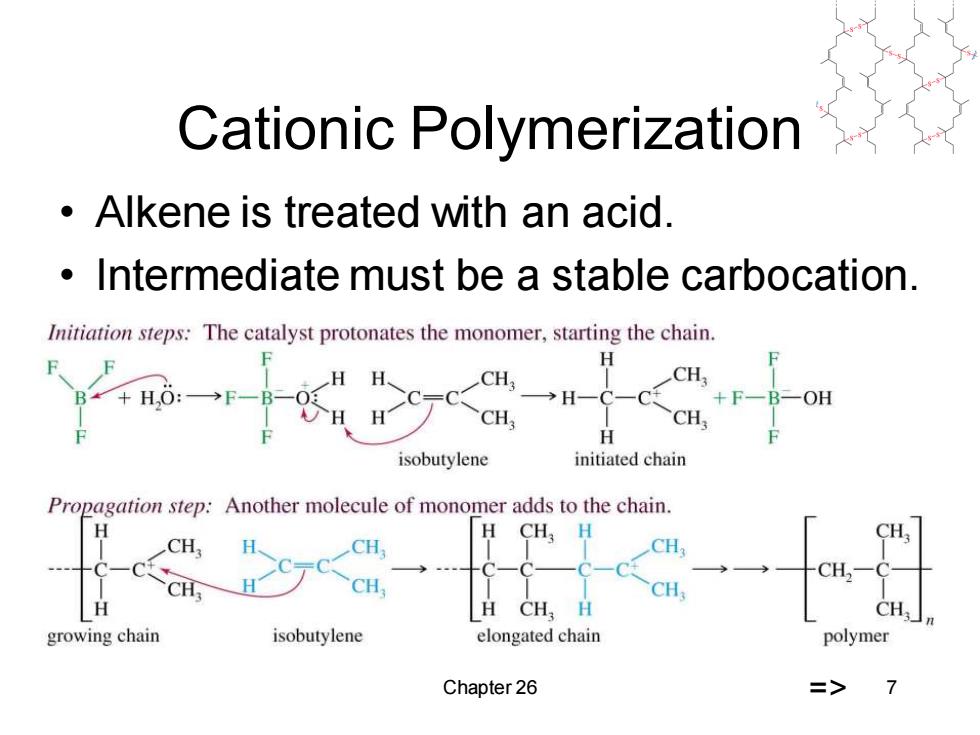
Cationic Polymerization Alkene is treated with an acid. Intermediate must be a stable carbocation. Initiation steps:The catalyst protonates the monomer,starting the chain. F H CH: B*+H,O:- CH; →H-C-C +FBOH CH, H isobutylene initiated chain Propagation step:Another molecule of monomer adds to the chain. H H CH,H CH CH CH CH,- CH CH CH H CH,H CH; growing chain isobutylene elongated chain polymer Chapter 26 => 7
Chapter 26 7 Cationic Polymerization • Alkene is treated with an acid. • Intermediate must be a stable carbocation. =>
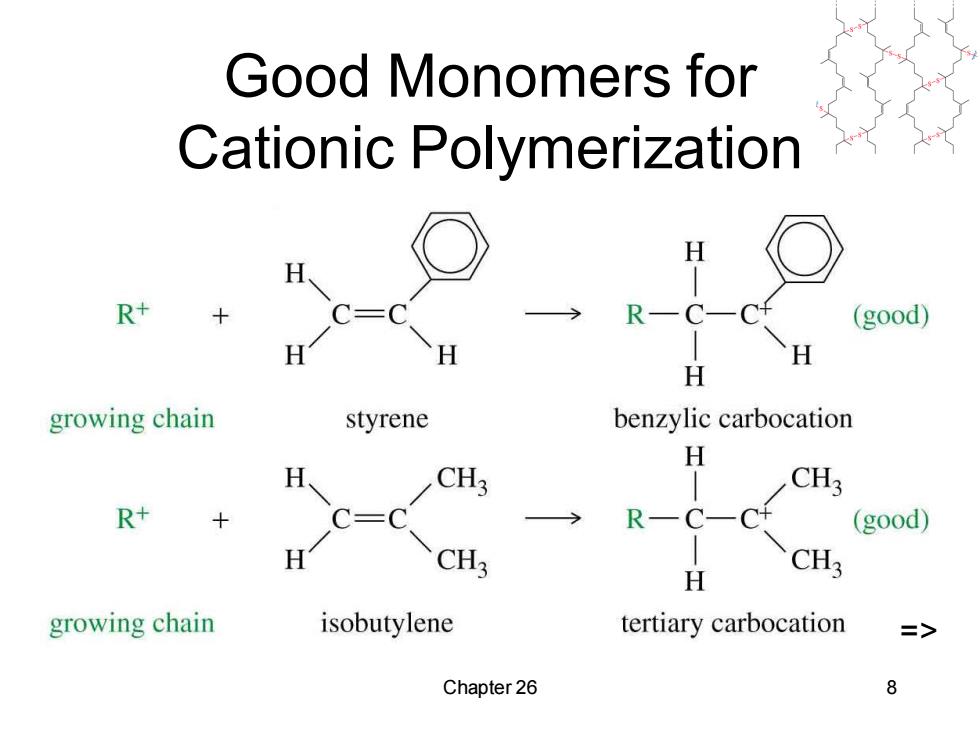
Good Monomers for Cationic Polymerization H R+ RC( (good) H H growing chain styrene benzylic carbocation H H CH3 CH3 R+ RCCH (good) CH3 H CH; growing chain isobutylene tertiary carbocation => Chapter 26 8
Chapter 26 8 Good Monomers for Cationic Polymerization =>
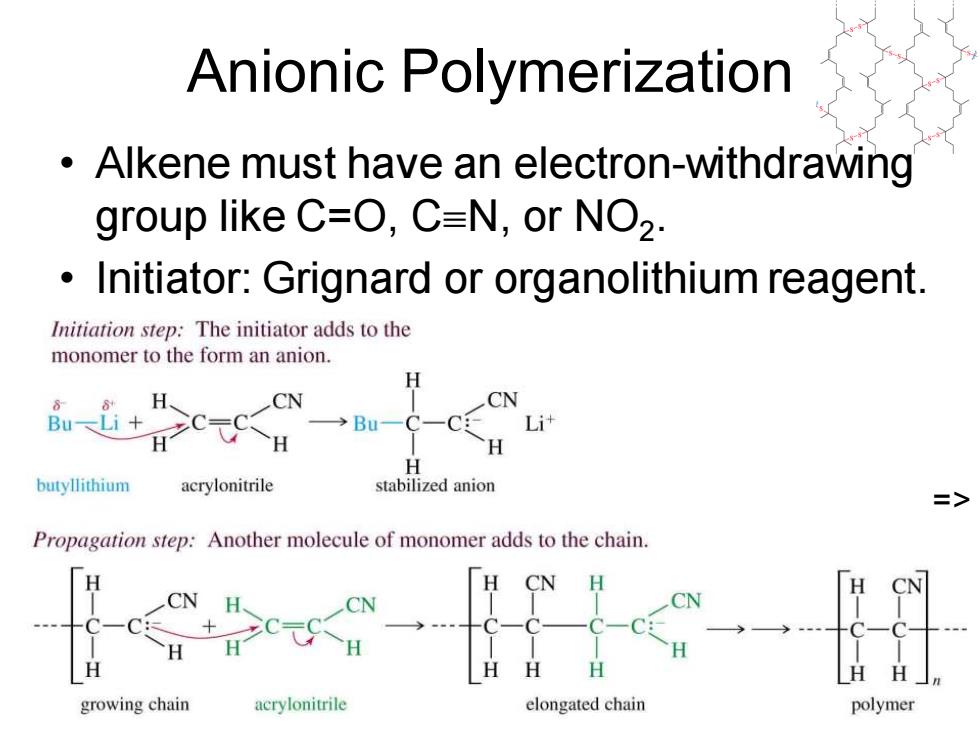
Anionic Polymerization Alkene must have an electron-withdrawing group like C=O,C=N,or NO2. Initiator:Grignard or organolithium reagent. Initiation step:The initiator adds to the monomer to the form an anion. H H CN CN Bu-Li+ →Bu Lit H H butyllithium acrylonitrile stabilized anion => Propagation step:Another molecule of monomer adds to the chain. CN H CN H H H HH」n growing chain acrylonitrile elongated chain polymer
Chapter 26 9 Anionic Polymerization • Alkene must have an electron-withdrawing group like C=O, CN, or NO2 . • Initiator: Grignard or organolithium reagent. =>