
第17章铜锌分族 Chapter 17 The copper 11B 12B 0 subgroup and zinc subgroup Cu Zn [Ar4s'3d10 [Ar4s23d10 copper zinc 63.55 65.39 copper subgroup: 47 8 Ag Copper,Silver,Gold 5 Cd [Kr]ss14d10 [Kr]5s24d10 silver cadmium 107.9 112.4 zinc subgroup: 79 0 Zinc,Cadmium,Mercury Au Hg Ke]6s14f145d10 Xe]6s24f145d10 gold mercury 197.0 200.5 (n-1)d10ns1 (n-1)d10ns2
第17章 铜锌分族 Chapter 17 The copper subgroup and zinc subgroup copper subgroup: Copper, Silver, Gold zinc subgroup: Zinc, Cadmium, Mercury (n-1)d10ns1 (n-1)d10ns2
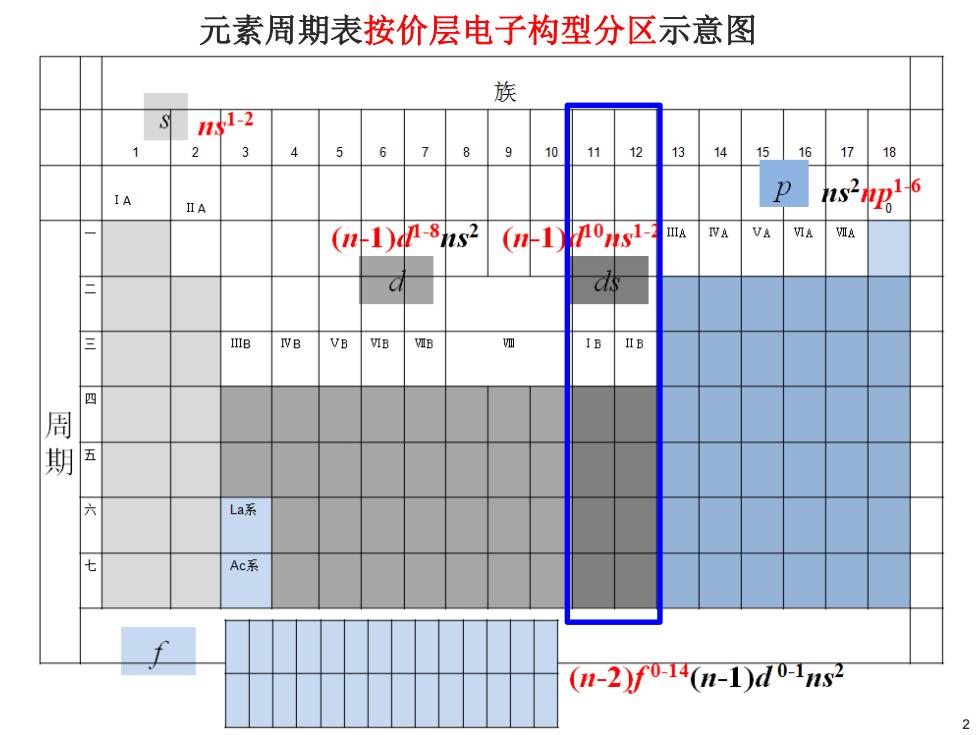
元素周期表按价层电子构型分区示意图 族 -2 1 2 45 67 89 10 1112 1314 1516 17 18 IA IIA s2阳6 (1)08s2 (n-1nons1 VA VIA VIA IVB VB VIB VIB 多 IB IIB 四 周 期 五 六 La系 七 Ac系 (-2f04n-1)d02s2
元素周期表按价层电子构型分区示意图 2
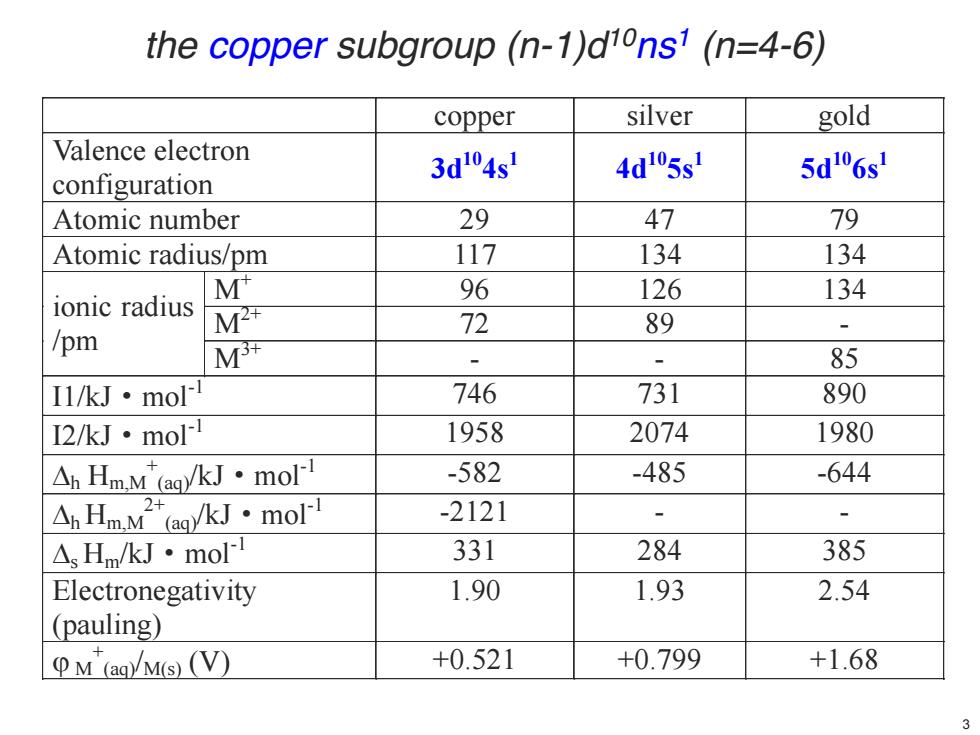
the copper subgroup(n-1)dions1(n=4-6) copper silver gold Valence electron 3d04s 4d05s 5d"6s configuration Atomic number 29 47 79 Atomic radius/pm 117 134 134 M 96 126 134 ionic radius M 72 89 /pm M - 、 85 I1/kJ·mol 746 731 890 I2/kJ·mol 1958 2074 1980 △Hm.M(/kJ·mold -582 -485 -644 AHmM2ekJ·mo -2121 、 △Hm/kJ·moll 331 284 385 Electronegativity 1.90 1.93 2.54 (pauling) M(agM(S)V) +0.521 +0.799 +1.68
copper silver gold Valence electron configuration 3d104s 1 4d105s 1 5d106s 1 Atomic number 29 47 79 Atomic radius/pm 117 134 134 ionic radius /pm M+ 96 126 134 M2+ 72 89 - M3+ - - 85 I1/kJ·mol-1 746 731 890 I2/kJ·mol-1 1958 2074 1980 'h Hm,M + (aq)/kJ·mol-1 -582 -485 -644 'h Hm,M 2+ (aq)/kJ·mol-1 -2121 - - 's Hm/kJ·mol-1 331 284 385 Electronegativity (pauling) 1.90 1.93 2.54 M M + (aq)/M(s) (V) +0.521 +0.799 +1.68 the copper subgroup (n-1)d10ns1 (n=4-6) 3
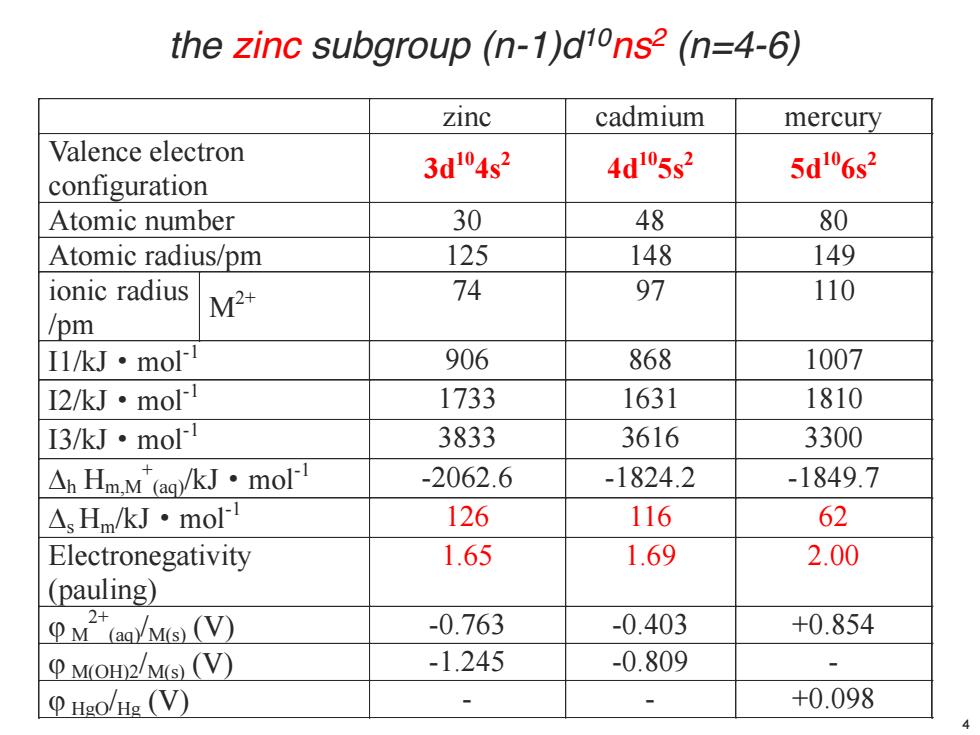
the zinc subgroup (n-1)d10ns2 (n=4-6) zinc cadmium mercury Valence electron 3d104s2 4d05s2 5d"6s2 configuration Atomic number 30 48 80 Atomic radius/pm 125 148 149 ionic radius M2+ 74 97 110 /pm I1/kJ·mol 906 868 1007 I2/kJ·mo 1733 1631 1810 I3/kJ·mo 3833 3616 3300 △Hm.M(ag)/kJ·moll -2062.6 -1824.2 -1849.7 △HmkJ·molI 126 116 62 Electronegativity 1.65 1.69 2.00 (pauling) 2+ PM(g)M(s)(V) -0.763 -0.403 +0.854 PMoH2Ms(V)) -1.245 -0.809 - P Hgo/Hg (V) 、 +0.098
zinc cadmium mercury Valence electron configuration 3d104s2 4d105s2 5d106s2 Atomic number 30 48 80 Atomic radius/pm 125 148 149 ionic radius /pm M2+ 74 97 110 I1/kJ·mol-1 906 868 1007 I2/kJ·mol-1 1733 1631 1810 I3/kJ·mol-1 3833 3616 3300 'h Hm,M + (aq)/kJ·mol-1 -2062.6 -1824.2 -1849.7 's Hm/kJ·mol-1 126 116 62 Electronegativity (pauling) 1.65 1.69 2.00 M M 2+ (aq)/M(s) (V) -0.763 -0.403 +0.854 M M(OH)2/M(s) (V) -1.245 -0.809 - M HgO/Hg (V) - - +0.098 the zinc subgroup (n-1)d10ns2 (n=4-6) 4
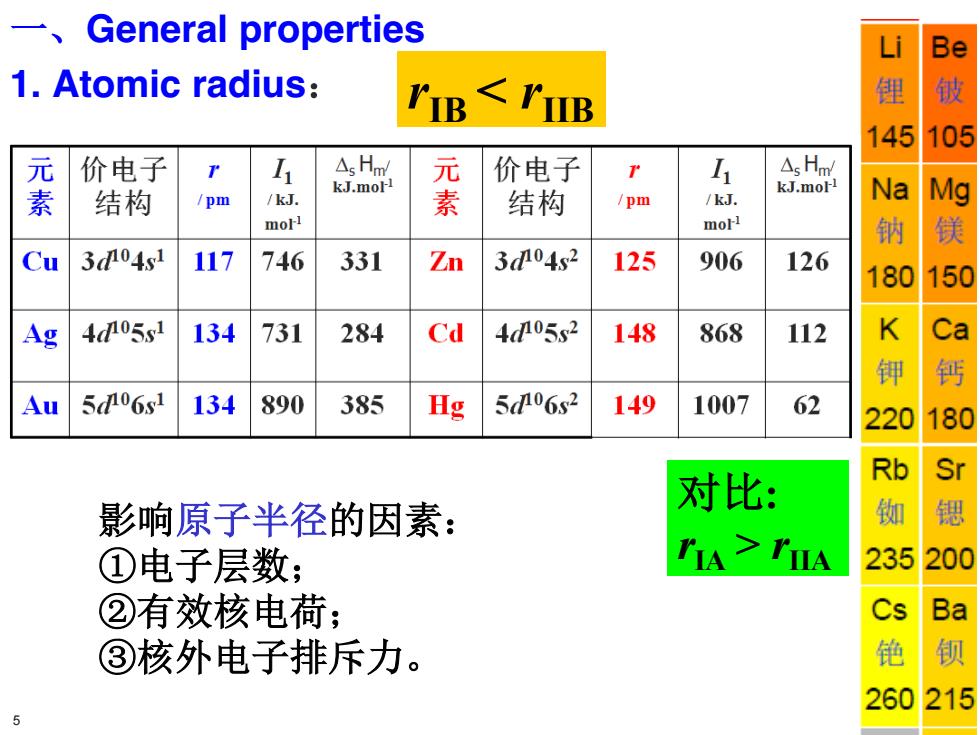
一、 General properties Li Be 1.Atomic radius: 锂 145 105 元 价电子 1 △sHn 价电子 r L △sHm 素 结构 kJ.mol /pm /kJ. 素 kJ.mol 结构 /pm /kJ. Na Mg mol1 morl 钠 Cu 3d04s1 117 746 331 Zn 304s2 125 906 126 180 150 Ag 405s1 134 731 284 Cd 405s2 148 868 112 K Ca 钾 钙 Au 5d06s1 134 890 385 Hg 506s2 149 1007 62 220 180 Rb Sr 对比: 影响原子半径的因素: ①电子层数; TIA>TIA 235 20 ②有效核电荷; Cs Ba ③核外电子排斥力。 铯 钡 260215
rIB < rIIB 对比: rIA > rIIA 影响原子半径的因素: ①电子层数; ②有效核电荷; ③核外电子排斥力。 一、General properties 1. Atomic radius: 5