
Lecture 7 196 Anode material for LIBs: Lithium Chen Junsong School of Materials and Energy 2018.10
Anode material for LIBs: Lithium Chen Junsong School of Materials and Energy 2018.10 Lecture 7

Content /986 ·Introduction of Li Advantage and disadvantage of Li Modification methods One literature example 2
2 Content • Introduction of Li • Advantage and disadvantage of Li • Modification methods • One literature example
Lithium 956 Periodi Atomic weight: 18 2 H 6.94 g/mol He 与m 16016- Lightest alkali metal 16 17 403 10 Li (0.54g/cm3) 0 F Ne en 64941 012 1R0用 11 Silvery,metallic solid 16 18 Na Mg Theoretical capacity: Ar Salfar 24305 6 > 35453 94 19 32 23 24 25 34 35 36 Ca Sc Cr Mn 3.86 Ah/g Se Br Kr Chroealam Nasganese 4007日 44956 50942 5196 5493细 E。=-3.04VsHE 2 7897 79904 37 39 40 41 43 52 53 Rb Sr Zr Nb Mo Tc Ru Ca m Sn Te Xe N比行n Rhodiam a向 Caduftm 4a 航2 995 9907 101.67 162 1078d 112.410 1101 11711 12170 12690 11224 55 57-71 72 73 74 75 76 78 79 80 83 84 85 86 Cs Ba Hf Ta Re r Pt Au Hg T Pb Bi At Rn ristes Pmith 1290 13n32 178.49 180.9号 1884 18207 190.23 19221 15 1696 200592 2043☒ 2072 2e980 289 2099 2218 87 89-103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db g Bh Hs Mt Ds Rg Cn Uut Lv Uus Uuo 0tt当端用 htn 222 226025 261 2 小w B9 57 58 60 61 62 63 64 65 66 67 68 69 70 71 a Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Sanarien Terbiam Hon当s Tbein T月a3un Lotetam 1905 140.116 140.90 1442日 144913 150.36 151964 15723 1925 160 164930 1629 16934 1725g 174967 89 90 93 94 95 96 98 99 100 101 102 103 AC. Th Np Am Cm Bk CE Es Fm Md No r Tortn a忙aem nengio Criin Beriellum Prlum 4otl柱与 2228 26 229 270梯 244064 243661 24070 207 2514 2的第 281 2511 lkal Metal Metal Batie Metal emimeta国 Nonmetal 3
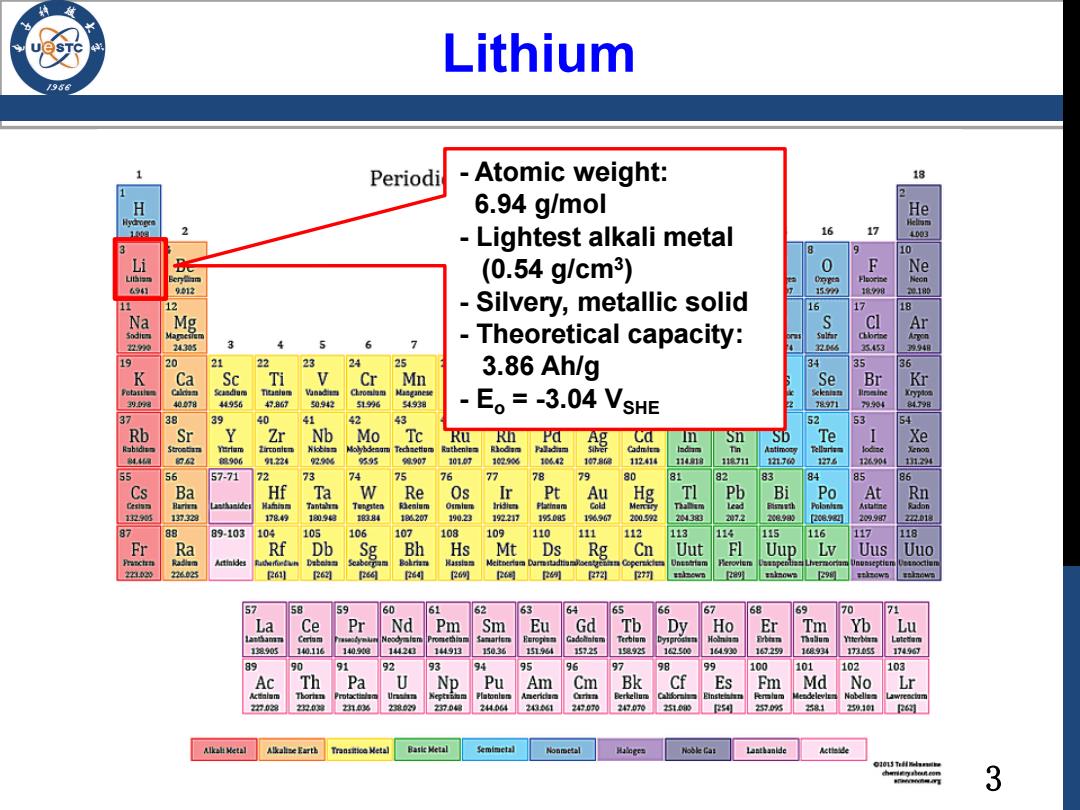
3 Lithium - Atomic weight: 6.94 g/mol - Lightest alkali metal (0.54 g/cm3 ) - Silvery, metallic solid - Theoretical capacity: 3.86 Ah/g - Eo = -3.04 VSHE

Advantage of Li /986 Li←→Lit+e The molecular weight of lithium is 6.94 g/mol,so its theoretical capacity is:96500:6.94-:3.6 3862.5 mA-h/g >Eo=-3.04 VsHE-high operating voltage of battery cells 4
4 Advantage of Li The molecular weight of lithium is 6.94 g/mol, so its theoretical capacity is: 96500÷6.94÷3.6 = 3862.5 mA·h/g Eo = -3.04 VSHE → high operating voltage of battery cells Li ↔ Li+ + e─ 1 2
Disadvantage of Li Formation of Li dendrite Solid electrolyte interphase(SEl) o LiLit@negative potential-electrolyte reduced at Li surface-passivation layer (SEl) Electrolyte:1 M LiPF6 in ethylene carbonate/diethyl carbonate (EC/DEC;1/1) Formation process: b Li dendrites Step 1 Step 2 Step3 Isolated Li Step 4 Cracks ]Dead Li Thick SEI SEl on Li Porous Li plating Further Li stripping electrode Continuous plating cycle Step 1:Li plating Step 2:further plating Step 3:Li stripping Step 4:Continuous cycling causes volume causes Li dendrites to produces isolated Li which causes steps 1-3 to occur expansion,which shoot out through the becomes part of the 'dead' repeatedly,and this finally cracks the SEl film. cracks. Li,while volume results in accumulated dead Li, contraction results in thick SEl and porous Li further SEl fracture. electrode 5 DO:10.1038/NNANO.2017.16
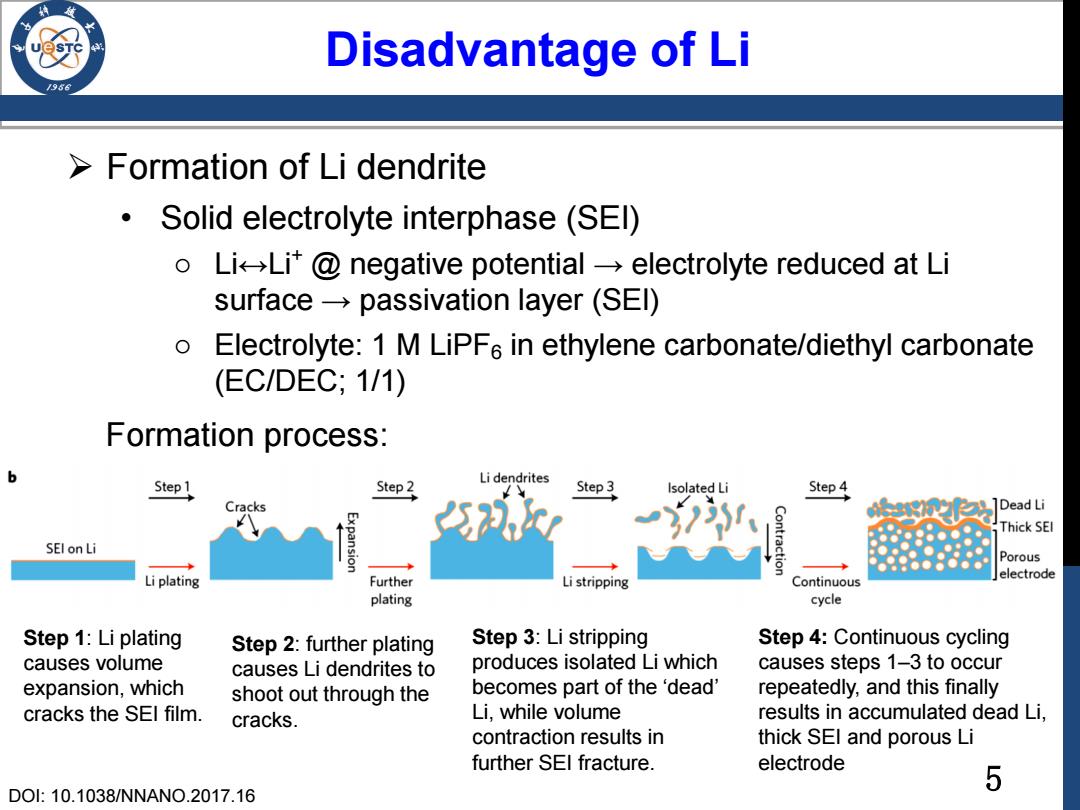
5 Disadvantage of Li Formation of Li dendrite • Solid electrolyte interphase (SEI) o Li↔Li+ @ negative potential → electrolyte reduced at Li surface → passivation layer (SEI) o Electrolyte: 1 M LiPF6 in ethylene carbonate/diethyl carbonate (EC/DEC; 1/1) Step 1: Li plating causes volume expansion, which cracks the SEI film. DOI: 10.1038/NNANO.2017.16 Step 2: further plating causes Li dendrites to shoot out through the cracks. Step 3: Li stripping produces isolated Li which becomes part of the ‘dead’ Li, while volume contraction results in further SEI fracture. Step 4: Continuous cycling causes steps 1–3 to occur repeatedly, and this finally results in accumulated dead Li, thick SEI and porous Li electrode Formation process: