
Lecture 4 1956 Cathode material for LIBs: LiFePO, Chen Junsong School of Materials and Energy 2020.04
Cathode material for LIBs: LiFePO4 Chen Junsong School of Materials and Energy 2020.04 Lecture 4

Content /986 Discovery ● Chemistry of LiFePO4 Molecular structure and Li diffusion Advantages and disadvantages Synthesis and modification methods One literature example 2
Content • Discovery • Chemistry of LiFePO4 • Molecular structure and Li diffusion • Advantages and disadvantages • Synthesis and modification methods • One literature example 2
Discovery Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries A.K.Padhi,'K.S.Nanjundaswamy,"and J.B.Goodenough Center for Materials Science and Engineering,The University of Texas at Austin,Austin,Texas 78712-1063,USA Reversible extraction of lithium from LiFePO,and insertion of lithium into FePO at 3.5 V vs.lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low-power,rechargeable lithium battery that is inexpensive,nontoxic, Akshaya Padhi J.B.Goodenough and environmentally benign. J.Electrochem.Soc.,Vol.144,No.4,April 1997 3
Discovery Akshaya Padhi J. B. Goodenough J. Electrochem. Soc., Vol. 144, No. 4, April 1997 Reversible extraction of lithium from LiFePO4 and insertion of lithium into FePO4 at 3.5 V vs. lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low-power, rechargeable lithium battery that is inexpensive, nontoxic, and environmentally benign. 3
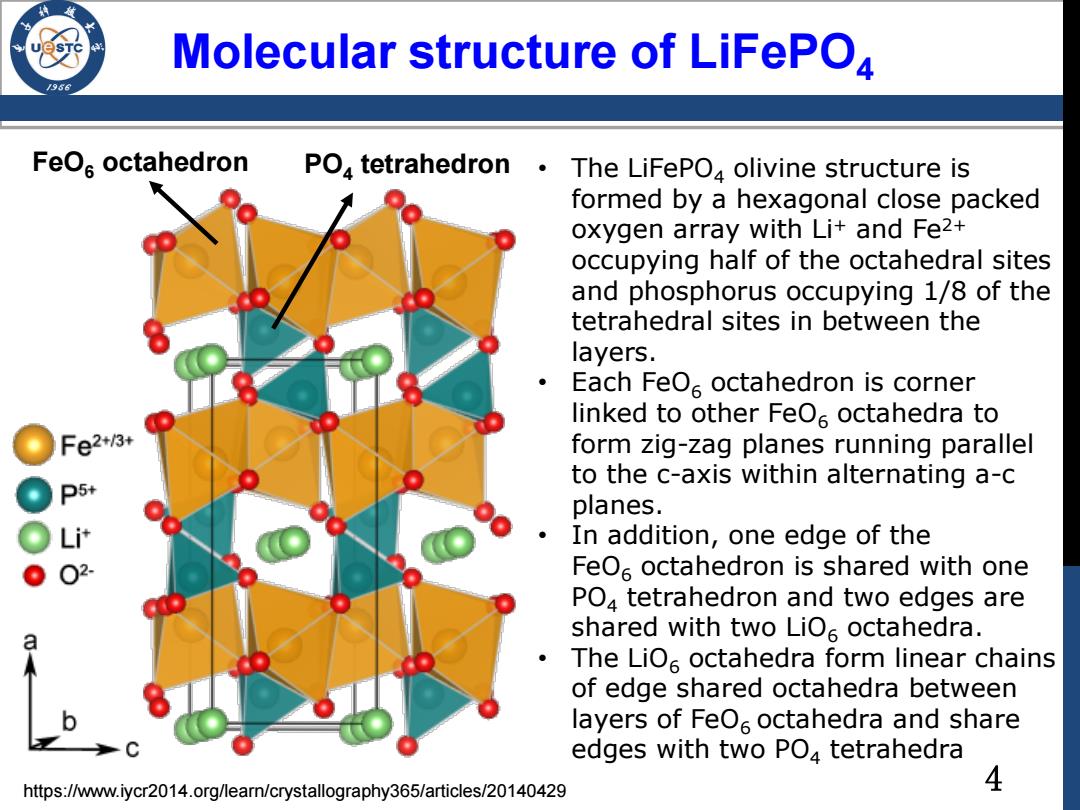
Molecular structure of LiFePO4 FeOs octahedron PO,tetrahedron· The LiFePO4 olivine structure is formed by a hexagonal close packed oxygen array with Li+and Fe2+ occupying half of the octahedral sites and phosphorus occupying 1/8 of the tetrahedral sites in between the layers. Each FeO6 octahedron is corner linked to other FeO6 octahedra to Fe2+/3+ form zig-zag planes running parallel to the c-axis within alternating a-c D5+ planes. Li计 In addition,one edge of the 02 FeOs octahedron is shared with one PO4 tetrahedron and two edges are shared with two LiOs octahedra. The LiO octahedra form linear chains of edge shared octahedra between layers of FeOs octahedra and share edges with two PO4 tetrahedra https://www.iycr2014.org/learn/crystallography365/articles/20140429 4
FeO6 octahedron PO4 tetrahedron https://www.iycr2014.org/learn/crystallography365/articles/20140429 Molecular structure of LiFePO4 • The LiFePO4 olivine structure is formed by a hexagonal close packed oxygen array with Li+ and Fe2+ occupying half of the octahedral sites and phosphorus occupying 1/8 of the tetrahedral sites in between the layers. • Each FeO6 octahedron is corner linked to other FeO6 octahedra to form zig-zag planes running parallel to the c-axis within alternating a-c planes. • In addition, one edge of the FeO6 octahedron is shared with one PO4 tetrahedron and two edges are shared with two LiO6 octahedra. • The LiO6 octahedra form linear chains of edge shared octahedra between layers of FeO6 octahedra and share edges with two PO4 tetrahedra 4

Li diffusion in LiFePO4 795 Li PO FeOe -Curved 0.8 ----Linear 0.6 0.4 0.2 E0s o 0 0.1 02 03 0.4 0.5 a Migration coordinate https://openi.nlm.nih.gov/imgs/512/396/4285883/PMC428 Chem.Mater.,Vol.17,No.20,2005 5883_m-02-00085-fig1.png Activation energy of Li+ Li+travels preferably along the [010] migration in LiFePO,shows direction,and follows a curved that it is much lower if Lit pathway,jumping through adjacent follows a Curved than a tetrahedral and octahedral voids. Linear path. 5
Li diffusion in LiFePO4 b a Li PO4 FeO6 Li+ travels preferably along the [010] direction, and follows a curved pathway, jumping through adjacent tetrahedral and octahedral voids. Activation energy of Li+ migration in LiFePO4 shows that it is much lower if Li+ follows a Curved than a Linear path. https://openi.nlm.nih.gov/imgs/512/396/4285883/PMC428 5883_m-02-00085-fig1.png Chem. Mater., Vol. 17, No. 20, 2005 5