
196 Anode material for LIB: TiO2 Chen Junsong School of Materials and Energy 2020.04
Anode material for LIB: TiO2 Chen Junsong School of Materials and Energy 2020.04
Content /986 ·Introduction of TiO2 。 Chemistry of TiO2 Molecular structure and Li insertion Synthesis and modification methods Several literature examples 2
2 Content • Introduction of TiO2 • Chemistry of TiO2 • Molecular structure and Li insertion • Synthesis and modification methods • Several literature examples
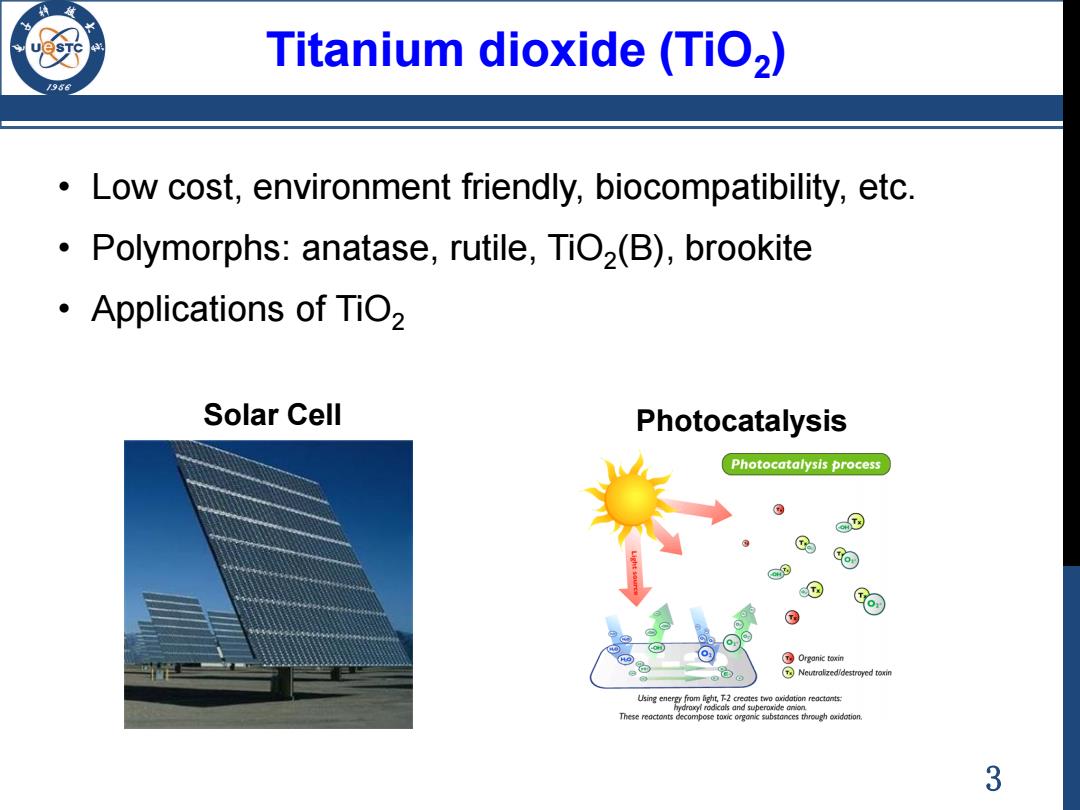
Titanium dioxide(TiO2) Low cost,environment friendly,biocompatibility,etc. Polymorphs:anatase,rutile,TiO(B),brookite ·Applications of TiO2 Solar Cell Photocatalysis Photocatalysis process @9 @ @ ⊙ Organic toxin Neuraizedidestroyed toin idation reactonts These reactonts decompose to through oidotion. 3
3 Titanium dioxide (TiO2 ) • Low cost, environment friendly, biocompatibility, etc. • Polymorphs: anatase, rutile, TiO2 (B), brookite • Applications of TiO2 Solar Cell Photocatalysis
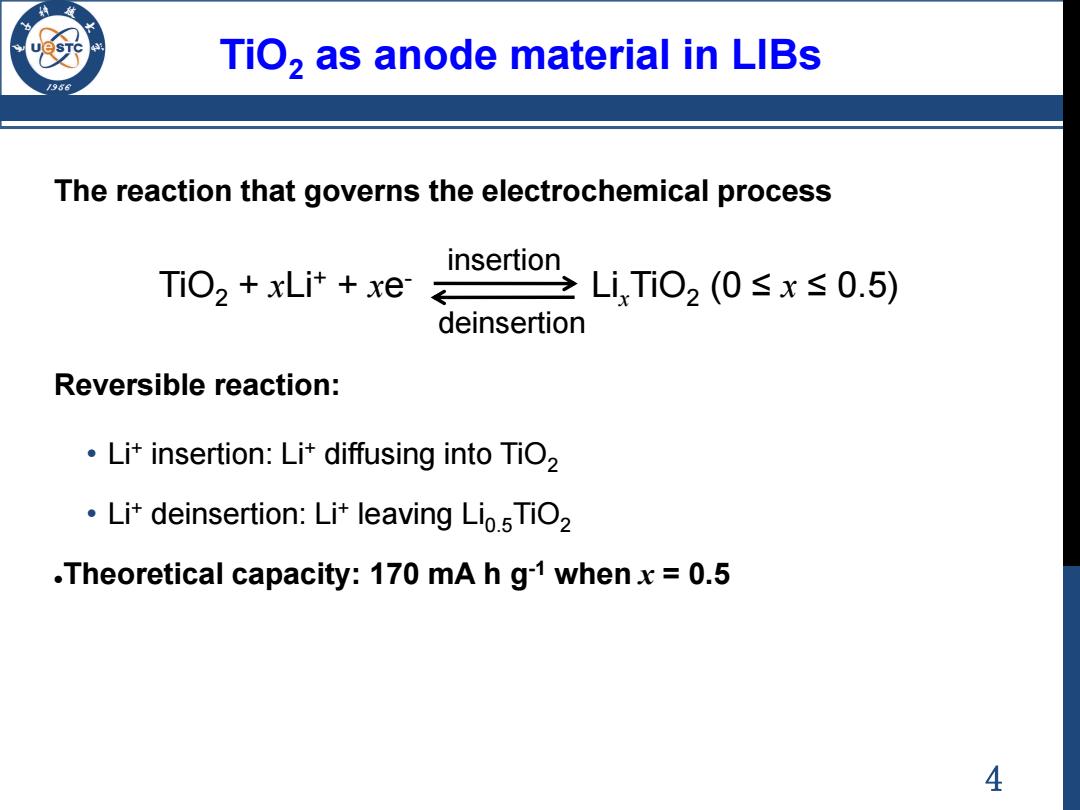
TiO,as anode material in LIBs 795 The reaction that governs the electrochemical process insertion TiO2 +xLit xe LiTi02(0≤x≤0.5) deinsertion Reversible reaction: Li+insertion:Lit diffusing into TiO2 Li+deinsertion:Lit leaving Lio.5TiO2 .Theoretical capacity:170 mA h g-1 whenx=0.5 4
4 TiO2 as anode material in LIBs The reaction that governs the electrochemical process TiO2 + xLi+ + xe - LixTiO2 (0 ≤ x ≤ 0.5) Reversible reaction: • Li+ insertion: Li+ diffusing into TiO2 • Li+ deinsertion: Li+ leaving Li0.5TiO2 Theoretical capacity: 170 mA h g-1 when x = 0.5 insertion deinsertion
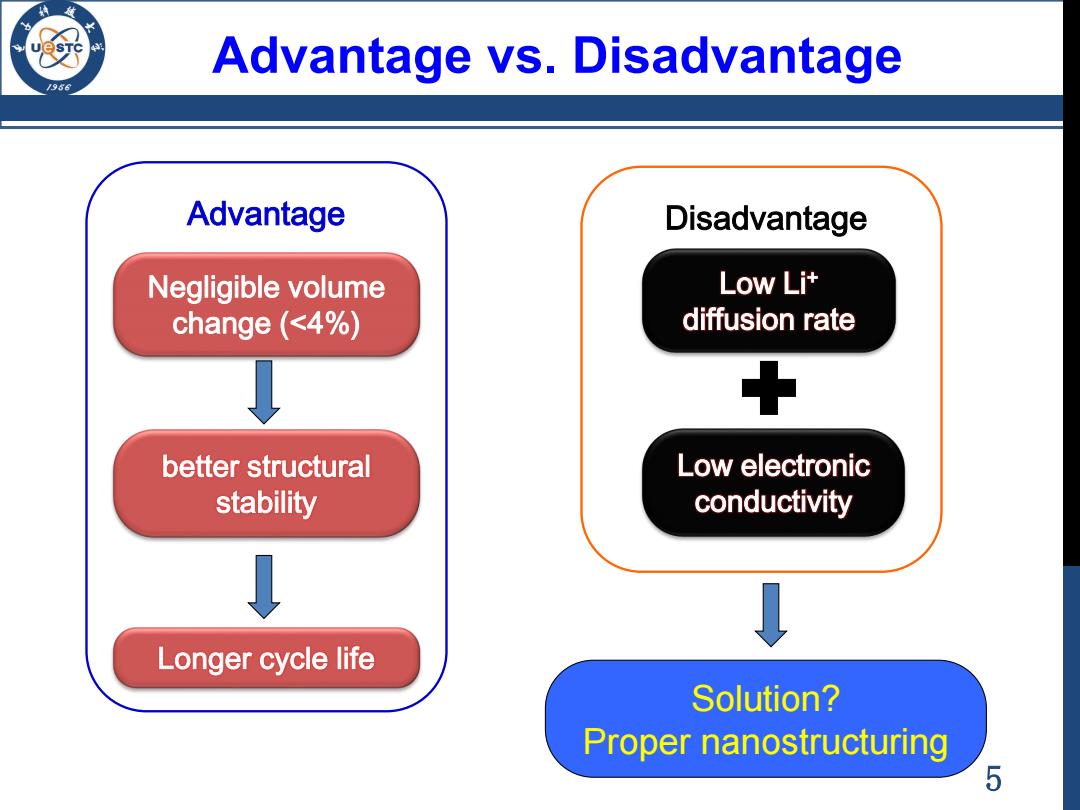
Advantage vs.Disadvantage 956 Advantage Disadvantage Negligible volume Low Li+ change (<4%) diffusion rate better structural Low electronic stability conductivity Longer cycle life Solution? Proper nanostructuring 5
5 Advantage vs. Disadvantage Negligible volume change (<4%) better structural stability Longer cycle life Advantage Solution? Proper nanostructuring Low Li+ diffusion rate Low electronic conductivity Disadvantage