
/936 Frontiers of Physical and Chemical Power Sources Part ll Chen Junsong School of Materials and Energy 2020
Chen Junsong School of Materials and Energy 2020 Frontiers of Physical and Chemical Power Sources Part II

196 Lecture 1 Background of Batteries 6
6 Lecture 1 Background of Batteries
Contents /986 ● Definition of battery >Working mechanism ●History of batteries Technology development Important types of batteries Characteristics,Comparisons 7
7 Contents Definition of battery Working mechanism History of batteries Technology development Important types of batteries Characteristics, Comparisons
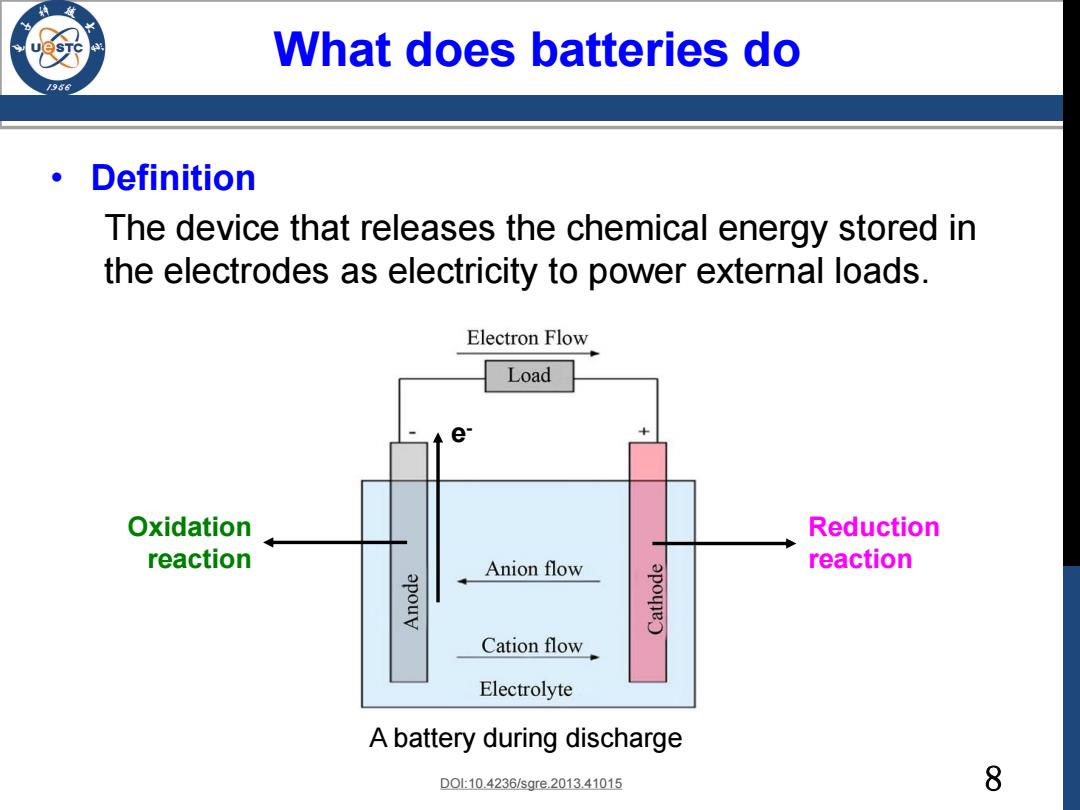
What does batteries do /98 Definition The device that releases the chemical energy stored in the electrodes as electricity to power external loads. Electron Flow Load Oxidation Reduction reaction Anion flow reaction Cation flow Electrolyte A battery during discharge D010.4236/sgre.2013.41015 8
8 What does batteries do • Definition The device that releases the chemical energy stored in the electrodes as electricity to power external loads. A battery during discharge DOI:10.4236/sgre.2013.41015 Oxidation reaction Reduction reaction e -
Battery Chemistry /98 Electrochemical reaction >A pair of redox reactions between elements at the two electrodes that involves the change of valence state > Oxidation at anode.reduction at cathode ●Free electrons > Free electrons are generated by the reactions at the electrodes ●Complete circuit > Electrons flow through the outer circuit to power up the external loads 9
9 Battery Chemistry Electrochemical reaction A pair of redox reactions between elements at the two electrodes that involves the change of valence state Oxidation at anode, reduction at cathode Free electrons Free electrons are generated by the reactions at the electrodes Complete circuit Electrons flow through the outer circuit to power up the external loads