
归东龙工大军 Analytical Chemistry (3)Dissociation of polyprotic acids and bases Acids with more than one acidic hydrogen ionize in steps.A dissociation constant expression may be written for each step.The stepwise dissociation and dissociation constant expression for a diprotic acid H,A are as follows: H,AU H+HA [H][A] [HAT 2022/10/26 12
Analytical Chemistry (3) Dissociation of polyprotic acids and bases Acids with more than one acidic hydrogen ionize in steps. A dissociation constant expression may be written for each step. The stepwise dissociation and dissociation constant expression for a diprotic acid H2A are as follows: 2022/10/26 12
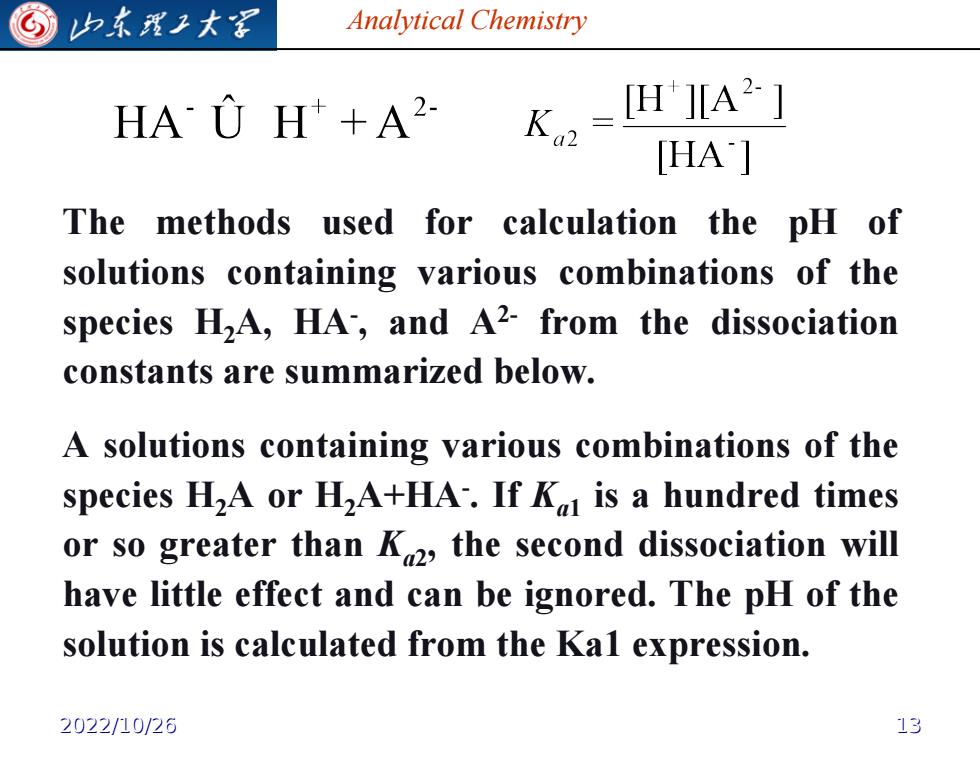
G 归东龙子大军 Analytical Chemistry HAU H+A2 K-H421 [HA'] The methods used for calculation the pH of solutions containing various combinations of the species HA,HA,and A2-from the dissociation constants are summarized below. A solutions containing various combinations of the species H2A or H2A+HA.If K is a hundred times or so greater than K2,the second dissociation will have little effect and can be ignored.The pH of the solution is calculated from the Kal expression. 2022/10/26 13
Analytical Chemistry The methods used for calculation the pH of solutions containing various combinations of the species H2A, HA- , and A2- from the dissociation constants are summarized below. A solutions containing various combinations of the species H2A or H2A+HA- . If Ka1 is a hundred times or so greater than Ka2 , the second dissociation will have little effect and can be ignored. The pH of the solution is calculated from the Ka1 expression. 2022/10/26 13
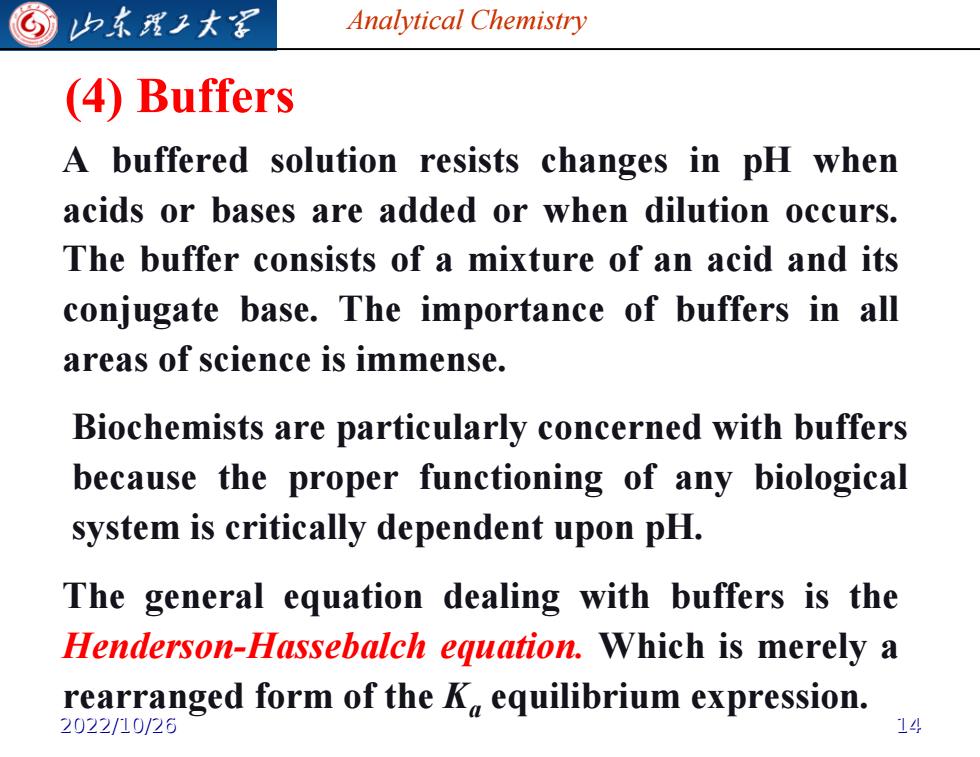
归东理工大军 Analytical Chemistry (4)Buffers A buffered solution resists changes in pH when acids or bases are added or when dilution occurs. The buffer consists of a mixture of an acid and its conjugate base.The importance of buffers in all areas of science is immense. Biochemists are particularly concerned with buffers because the proper functioning of any biological system is critically dependent upon pH. The general equation dealing with buffers is the Henderson-Hassebalch equation.Which is merely a rearranged form of the K equilibrium expression. 2022/10/26
Analytical Chemistry (4) Buffers A buffered solution resists changes in pH when acids or bases are added or when dilution occurs. The buffer consists of a mixture of an acid and its conjugate base. The importance of buffers in all areas of science is immense. Biochemists are particularly concerned with buffers because the proper functioning of any biological system is critically dependent upon pH. The general equation dealing with buffers is the Henderson-Hassebalch equation. Which is merely a rearranged form of the Ka equilibrium expression. 2022/10/26 14
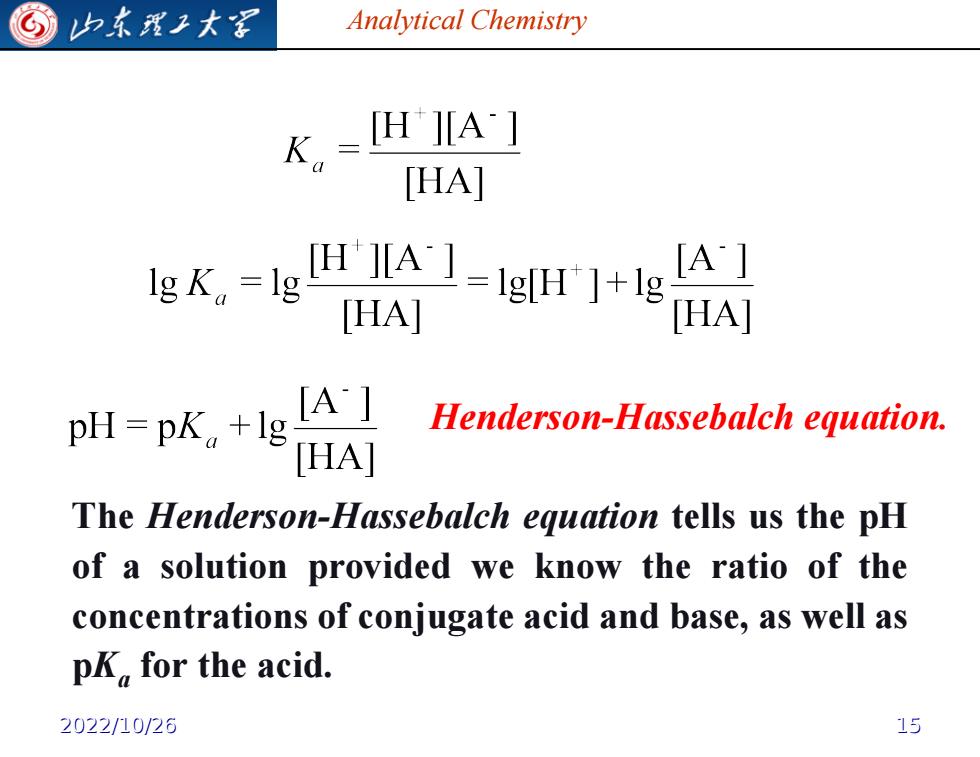
G 归东理子大图 Analytical Chemistry K,=IHHA:1 [HA] lgK。=lg H1-l [HAI HA pH-pk,+lgAI Henderson-Hassebalch equation. HAI The Henderson-Hassebalch equation tells us the pH of a solution provided we know the ratio of the concentrations of conjugate acid and base,as well as pK,for the acid. 2022/10/26 15
Analytical Chemistry Henderson-Hassebalch equation. The Henderson-Hassebalch equation tells us the pH of a solution provided we know the ratio of the concentrations of conjugate acid and base, as well as pKa for the acid. 2022/10/26 15
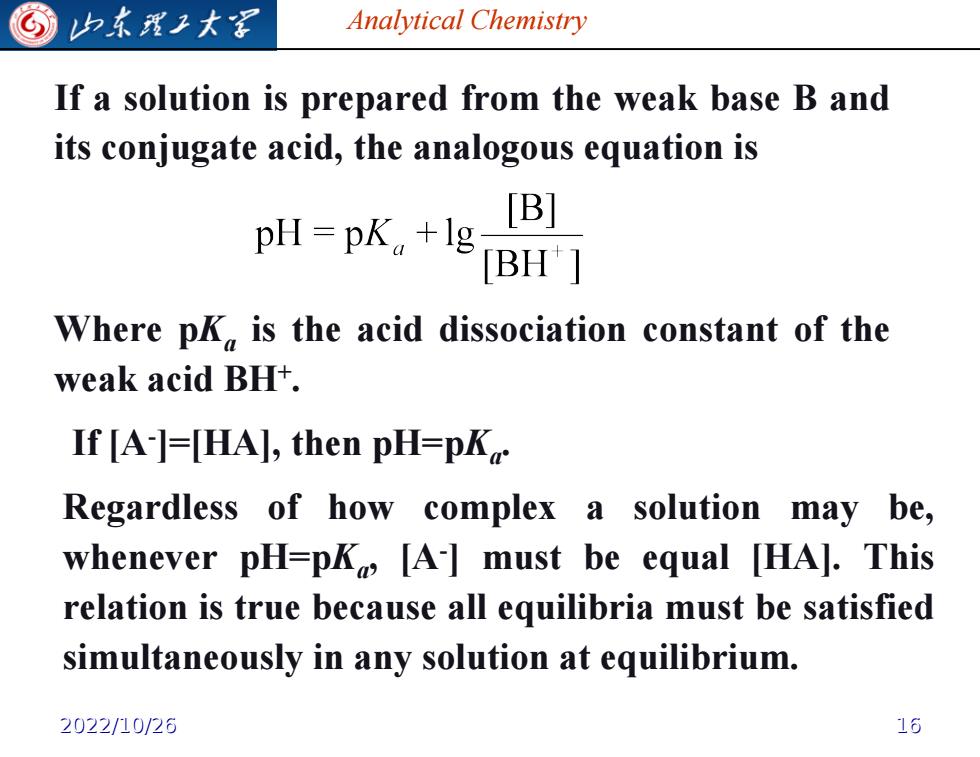
归东理工大军 Analytical Chemistry If a solution is prepared from the weak base B and its conjugate acid,the analogous equation is pH=pK。+lg [B] BH'] Where pK is the acid dissociation constant of the weak acid BH+. If [A-]=[HA],then pH=pK Regardless of how complex a solution may be, whenever pH=pk [A-]must be equal [HA].This relation is true because all equilibria must be satisfied simultaneously in any solution at equilibrium. 2022/10/26 16
Analytical Chemistry If a solution is prepared from the weak base B and its conjugate acid, the analogous equation is Where pKa is the acid dissociation constant of the weak acid BH+ . If [A- ]=[HA], then pH=pKa . Regardless of how complex a solution may be, whenever pH=pKa , [A- ] must be equal [HA]. This relation is true because all equilibria must be satisfied simultaneously in any solution at equilibrium. 2022/10/26 16