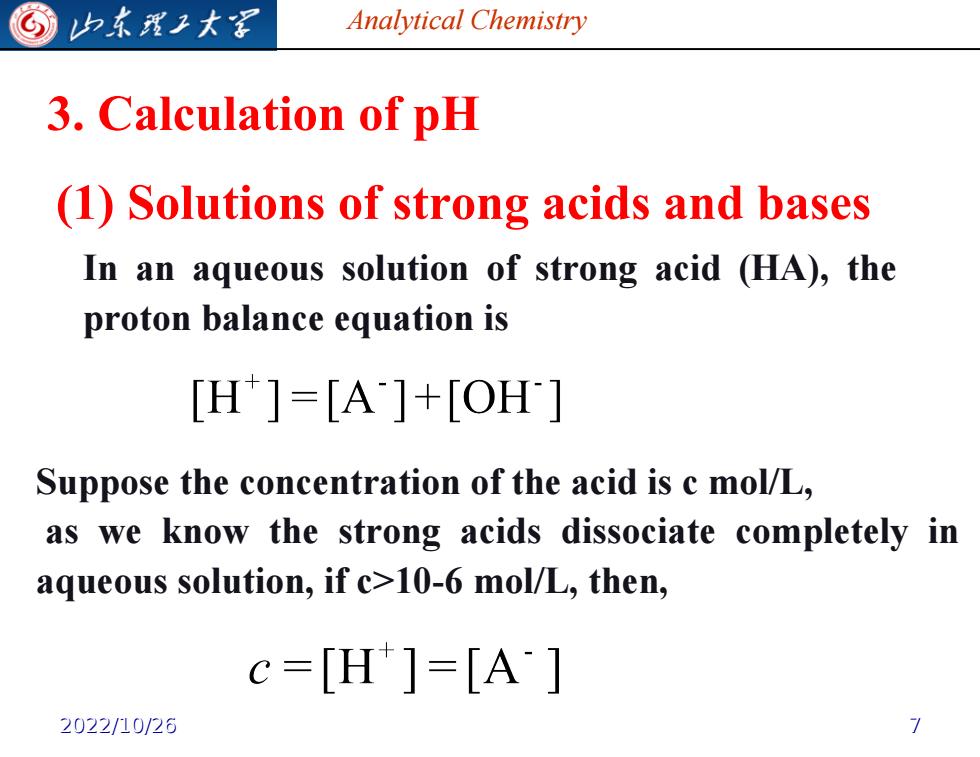
G 归东我子大军 Analytical Chemistry 3.Calculation of pH (1)Solutions of strong acids and bases In an aqueous solution of strong acid (HA),the proton balance equation is [H]=[A]+[OH] Suppose the concentration of the acid is c mol/L, as we know the strong acids dissociate completely in aqueous solution,if c>10-6 mol/L,then, c=[H]=[A] 2022/10/26
Analytical Chemistry 3. Calculation of pH (1) Solutions of strong acids and bases In an aqueous solution of strong acid (HA), the proton balance equation is Suppose the concentration of the acid is c mol/L, as we know the strong acids dissociate completely in aqueous solution, if c>10-6 mol/L, then, 2022/10/26 7
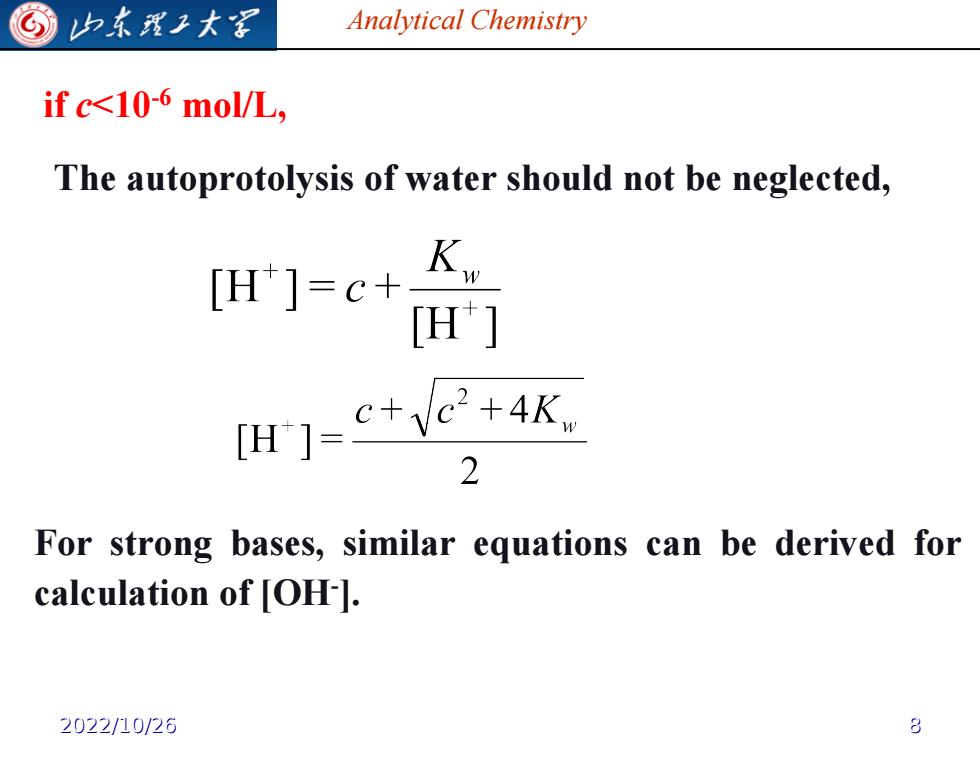
归东龙工大军 Analytical Chemistry if c<10-6 mol/L, The autoprotolysis of water should not be neglected, [H]=c+ H] [H1=c+e+4K 2 For strong bases,similar equations can be derived for calculation of [OH-]. 2022/10/26
Analytical Chemistry if c<10-6 mol/L, The autoprotolysis of water should not be neglected, For strong bases, similar equations can be derived for calculation of [OH- ]. 2022/10/26 8

G 归东理子大图 Analytical Chemistry (2)Solutions of weak acids and bases The pH of a weak acid can be calculated from the dissociation constant,K.Similarly,the pH of a weak base can be calculated from its Kp value. For a strong bases,similar equations can be derived for calculation of [OH-]. For a weak acid,HA,we can write its proton balance equation: H]=[A]+[OH] 2022/10/26
Analytical Chemistry (2) Solutions of weak acids and bases The pH of a weak acid can be calculated from the dissociation constant, Ka . Similarly, the pH of a weak base can be calculated from its Kb value. For a strong bases, similar equations can be derived for calculation of [OH- ]. For a weak acid, HA, we can write its proton balance equation: 2022/10/26 9

归东理工大 Analytical Chemistry H']= Ko[HA]K [H] [H] We obtain [H']=K[HA]+K If K,is not extremely small,that is cK。320K The K can be neglected,so [H']K[HA]=K.(e-[H']) H=K+K+4K,c 2 2022/10/26 10
Analytical Chemistry We obtain If Ka is not extremely small, that is The Kw can be neglected, so 2022/10/26 10

G 归东龙子大图 Analytical Chemistry If c/K,≥400,the concentration of HA can be considered c,the analytical concentration of the weak acid,then [H']=Kc This is the simplified equation. Similarly,the simplified equation of a weak base concentration is [OH ]=K,c 2022/10/26 11
Analytical Chemistry If c/Ka ≥400, the concentration of HA can be considered c, the analytical concentration of the weak acid, then This is the simplified equation. Similarly, the simplified equation of a weak base concentration is 2022/10/26 11