
归东理工大军 Analytical Chemistry To master the requirements of the titration error; To know about the classical applications and calculation of all kinds of titrations. 2022/10/26 2
Analytical Chemistry ØTo master the requirements of the titration error; Ø To know about the classical applications and calculation of all kinds of titrations. 2022/10/26 2

G 归东龙子大图 Analytical Chemistry $4.1 Acid-base equilibria 1.Acid-base theory The acidity or basicity of a solution is frequently an important factor in chemical reactions.Fundamental acid-base equilibria are important in understanding acid-base titration and the effect of acids on chemical species and reactions. According to Bronsted theory: An acid is a substance that can give up protons; A base is any compound or ion that can accept protons. 2022/10/26
Analytical Chemistry §4.1 Acid-base equilibria 1. Acid-base theory The acidity or basicity of a solution is frequently an important factor in chemical reactions. Fundamental acid-base equilibria are important in understanding acid-base titration and the effect of acids on chemical species and reactions. According to Bronsted theory: ØAn acid is a substance that can give up protons; ØA base is any compound or ion that can accept protons. 2022/10/26 3

归东理工大军 Analytical Chemistry A strong acid or base is completely dissociated in aqueous solution; A weak acid is one that is only partially dissociated in water,all weak acids HA react with water by donating a proton to h2O: HA+H,OU H.O+A The acid dissociation constant is K,H][A】 [HA] 2022/10/26
Analytical Chemistry ØA strong acid or base is completely dissociated in aqueous solution; ØA weak acid is one that is only partially dissociated in water, all weak acids HA react with water by donating a proton to H2O: The acid dissociation constant is 2022/10/26 4
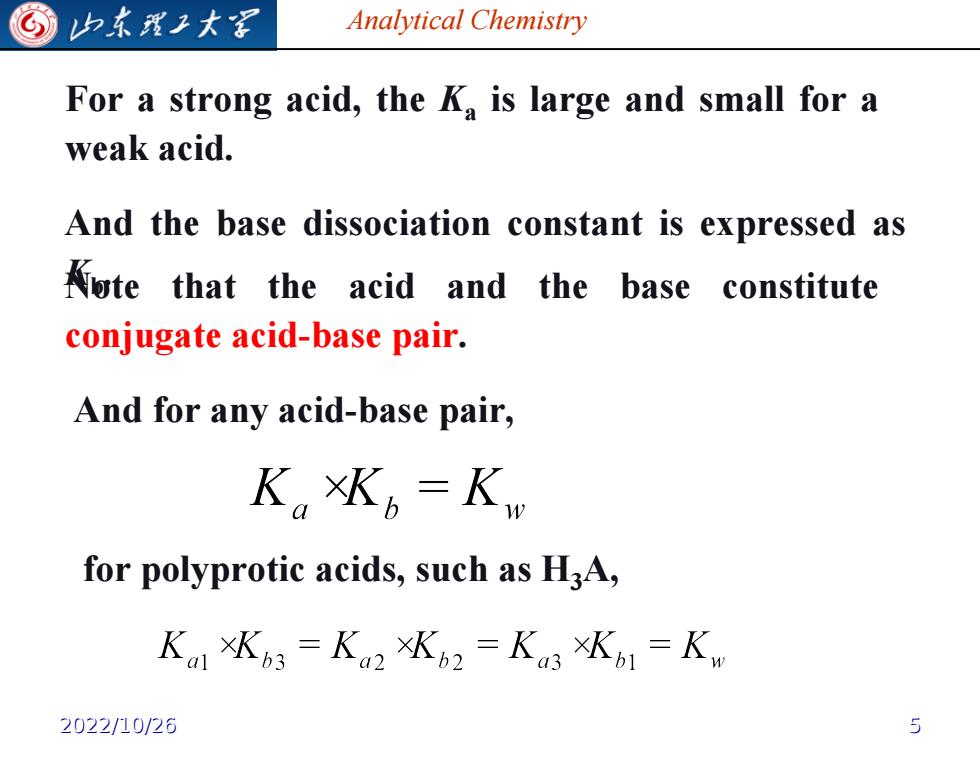
G 归东理子大溪 Analytical Chemistry For a strong acid,the k is large and small for a weak acid. And the base dissociation constant is expressed as Aute that the acid and the base constitute conjugate acid-base pair. And for any acid-base pair, K。Kb=K for polyprotic acids,such as H3A, Kal xKb3 =Ka2 xKb2 =Ka3 XKo1=Kw 2022/10/26 5
Analytical Chemistry For a strong acid, the Ka is large and small for a weak acid. And the base dissociation constant is expressed as Kb Note . that the acid and the base constitute conjugate acid-base pair. And for any acid-base pair, for polyprotic acids, such as H3A, 2022/10/26 5

归东龙工大军 Analytical Chemistry 2.Proton balance equation Acid-base reactions essentially involve the proton transferring process. And the number of protons that the acid gives up is always equal to that the base accepts. For example,in a Na,CO3 solution,the proton balance equation is: [H]+[HCO3]+2[HCO3]=[OH] For c mol/LNaH2PO solution, PBE [H]+〔H3P04)=(HP042-)+2P043-)+〔OH) 2022/10/26
Analytical Chemistry 2. Proton balance equation Acid-base reactions essentially involve the proton transferring process. And the number of protons that the acid gives up is always equal to that the base accepts. For example, in a Na2CO3 solution, the proton balance equation is: For c mol/LNaH2PO4 solution, PBE [H+ ]+ 〔H3PO4〕 = 〔HPO4 2-〕+ 2[PO4 3-〕 +〔OH-〕 2022/10/26 6