
G归东理王大军 Analytical Chemistry Chapter 5 Complexometric Titration Learning objectives >To master the fundamental principles and relevant calculations about complex equilibrium; >To master the calculation of titration curve,the end point and the titration and the titration break; >To understand the calculation of titration error; >To know about the application of complexometric titr
Analytical Chemistry 1 Chapter 5 Complexometric Titration Learning objectives ➢To master the fundamental principles and relevant calculations about complex equilibrium; ➢To master the calculation of titration curve, the end point and the titration and the titration break; ➢To understand the calculation of titration error; ➢To know about the application of complexometric titration
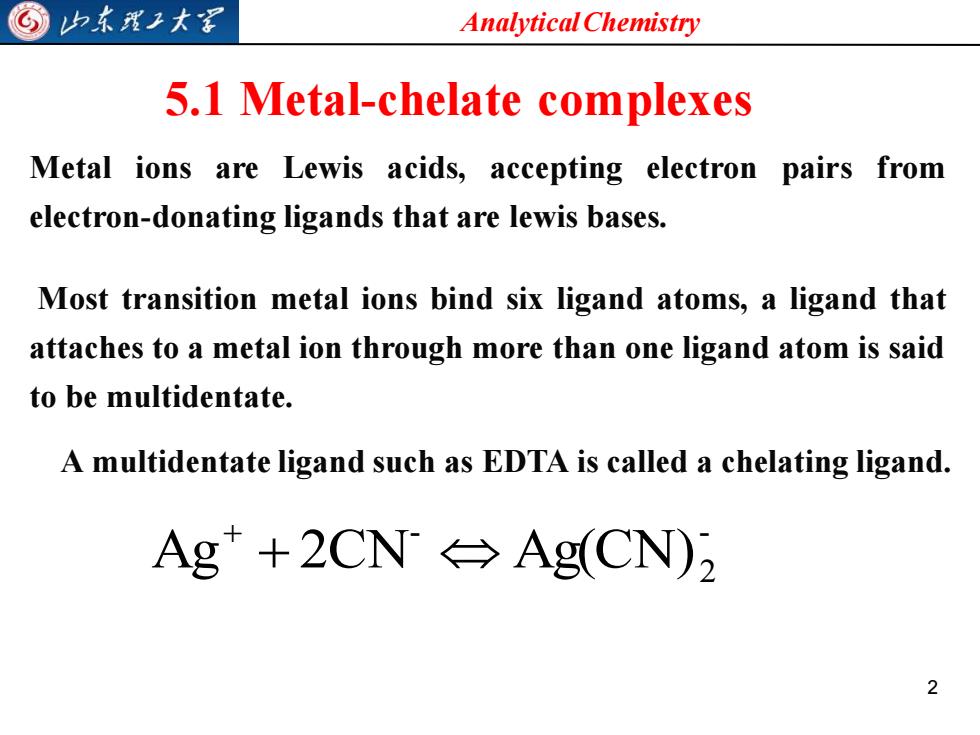
©山东理王大军 Analytical Chemistry 5.1 Metal-chelate complexes Metal ions are Lewis acids,accepting electron pairs from electron-donating ligands that are lewis bases. Most transition metal ions bind six ligand atoms,a ligand that attaches to a metal ion through more than one ligand atom is said to be multidentate. A multidentate ligand such as EDTA is called a chelating ligand. Ag+2CN→Ag(CN)2 2
Analytical Chemistry 2 5.1 Metal-chelate complexes Metal ions are Lewis acids, accepting electron pairs from electron-donating ligands that are lewis bases. Most transition metal ions bind six ligand atoms, a ligand that attaches to a metal ion through more than one ligand atom is said to be multidentate. A multidentate ligand such as EDTA is called a chelating ligand. - 2 - Ag + 2CN Ag(CN) +
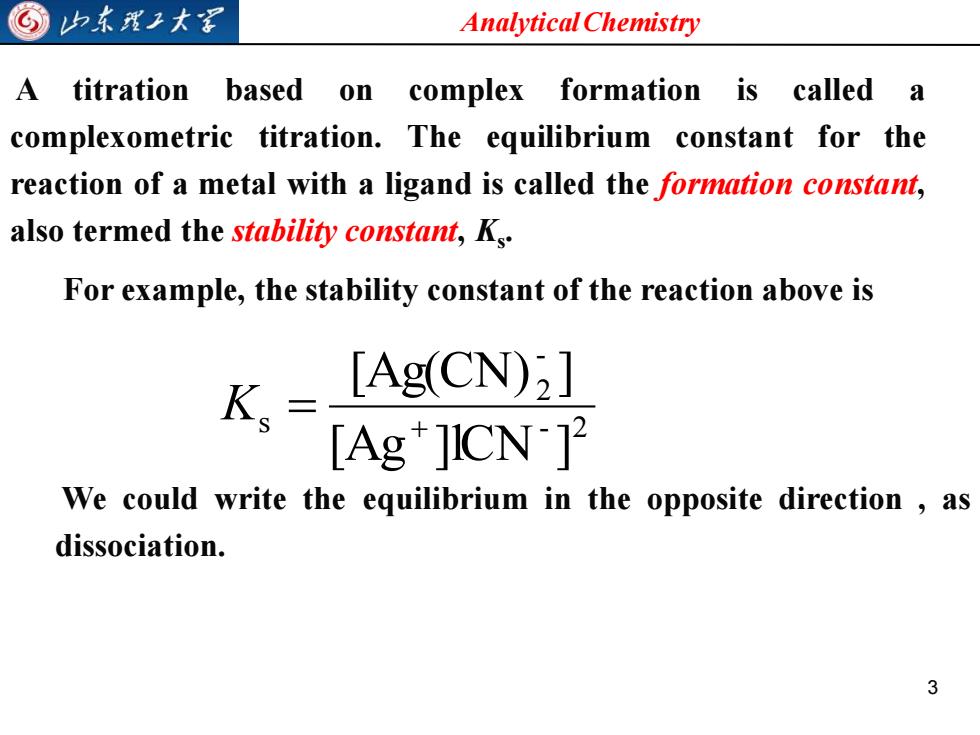
⑤归东理工大 Analytical Chemistry A titration based on complex formation is called a complexometric titration.The equilibrium constant for the reaction of a metal with a ligand is called the formation constant, also termed the stability constant,Ks. For example,the stability constant of the reaction above is Ks= [Ag(CN) [Ag+JICN] We could write the equilibrium in the opposite direction as dissociation. 3
Analytical Chemistry 3 A titration based on complex formation is called a complexometric titration. The equilibrium constant for the reaction of a metal with a ligand is called the formation constant, also termed the stability constant, Ks . For example, the stability constant of the reaction above is - 2 - 2 s [Ag ]lCN ] [Ag(CN) ] + K = We could write the equilibrium in the opposite direction , as dissociation
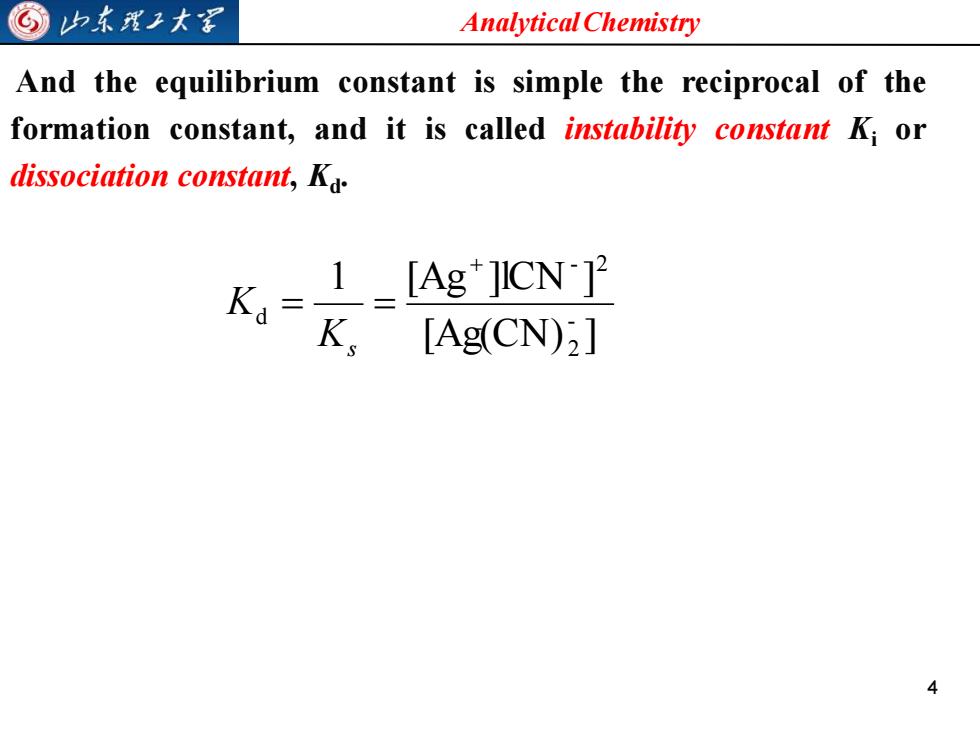
G山东我王大军 Analytical Chemistry And the equilibrium constant is simple the reciprocal of the formation constant,and it is called instability constant Ki or dissociation constant,Ka _1_[Ag*JICN K=[Ag(CN):]
Analytical Chemistry 4 And the equilibrium constant is simple the reciprocal of the formation constant, and it is called instability constant Ki or dissociation constant, Kd . [Ag(CN) ] 1 [Ag ]lCN ] - 2 - 2 d + = = Ks K
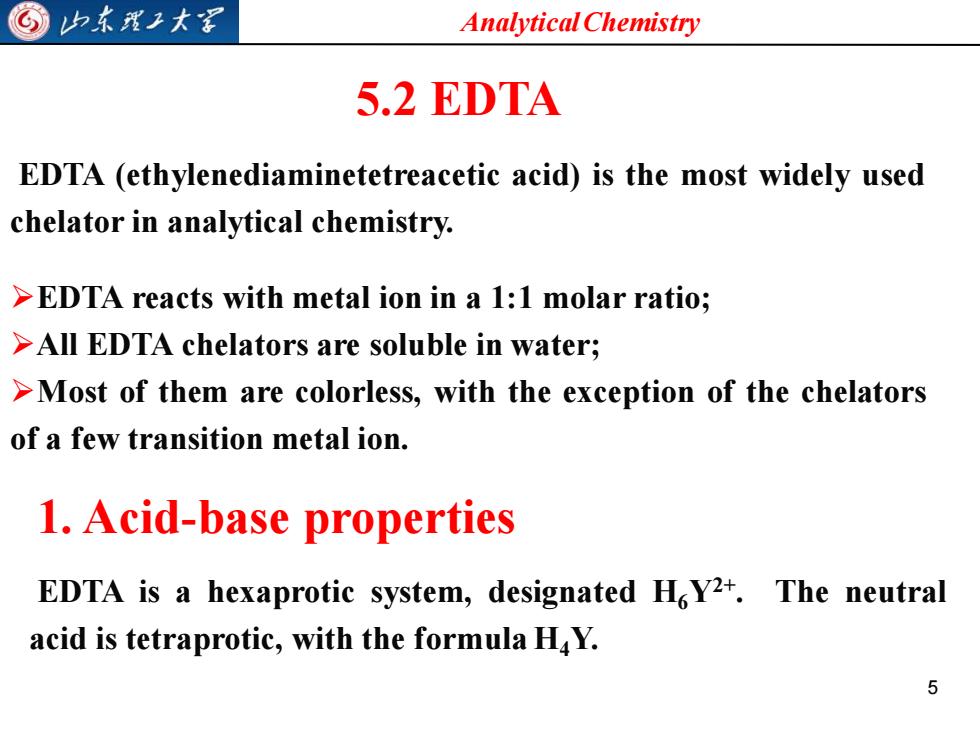
G归东理王大军 Analytical Chemistry 5.2 EDTA EDTA (ethylenediaminetetreacetic acid)is the most widely used chelator in analytical chemistry. >EDTA reacts with metal ion in a 1:1 molar ratio; >All EDTA chelators are soluble in water; >Most of them are colorless,with the exception of the chelators of a few transition metal ion. 1.Acid-base properties EDTA is a hexaprotic system,designated HoY2+.The neutral acid is tetraprotic,with the formula H4Y. 5
Analytical Chemistry 5 5.2 EDTA EDTA (ethylenediaminetetreacetic acid) is the most widely used chelator in analytical chemistry. ➢EDTA reacts with metal ion in a 1:1 molar ratio; ➢All EDTA chelators are soluble in water; ➢Most of them are colorless, with the exception of the chelators of a few transition metal ion. 1. Acid-base properties EDTA is a hexaprotic system, designated H6Y2+ . The neutral acid is tetraprotic, with the formula H4Y