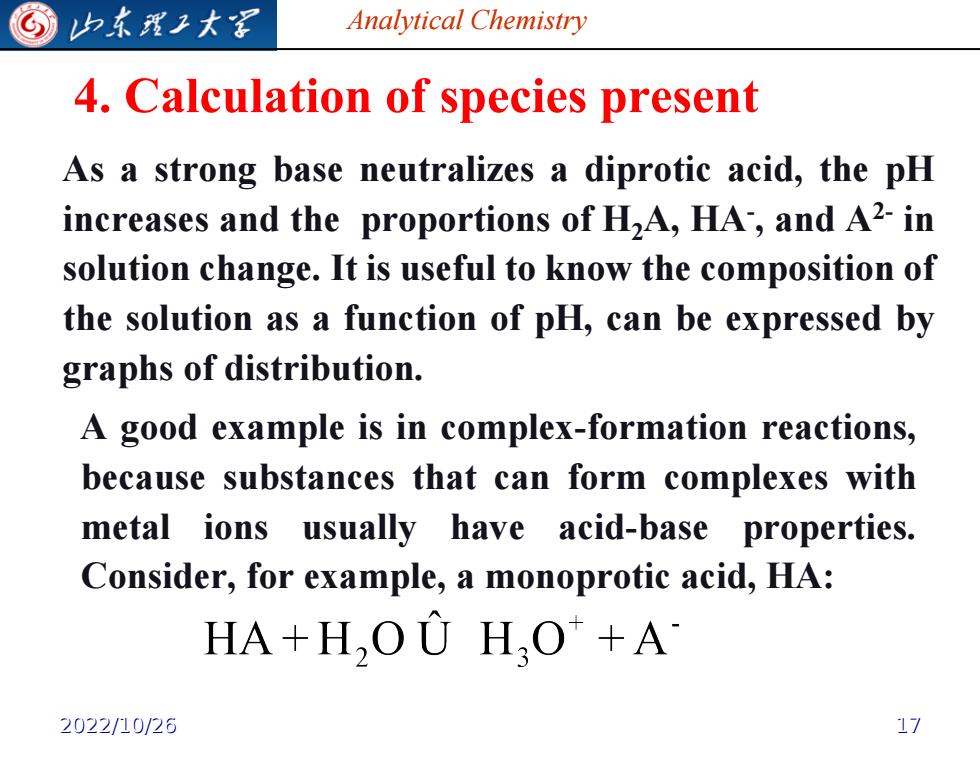
G 归东理子大军 Analytical Chemistry 4.Calculation of species present As a strong base neutralizes a diprotic acid,the pH increases and the proportions of HA,HA,and A2-in solution change.It is useful to know the composition of the solution as a function of pH,can be expressed by graphs of distribution. A good example is in complex-formation reactions, because substances that can form complexes with metal ions usually have acid-base properties. Consider,for example,a monoprotic acid,HA: HA+H,OU HO+A 2022/10/26 17
Analytical Chemistry 4. Calculation of species present As a strong base neutralizes a diprotic acid, the pH increases and the proportions of H2A, HA- , and A2- in solution change. It is useful to know the composition of the solution as a function of pH, can be expressed by graphs of distribution. A good example is in complex-formation reactions, because substances that can form complexes with metal ions usually have acid-base properties. Consider, for example, a monoprotic acid, HA: 2022/10/26 17

归东理工大军 Analytical Chemistry The analytical concentration of the monoprotic acid is c=[HA]+[A] c=[HA](1+ [H1 We use HA to express the fraction HA in the total concentration.The fraction is defined as distribution coefficient. du =HA] 1 [H] 1+K。 [H]+K。 [H] 2022/10/26 18
Analytical Chemistry The analytical concentration of the monoprotic acid is We use δHA to express the fraction HA in the total concentration. The fraction is defined as distribution coefficient. 2022/10/26 18
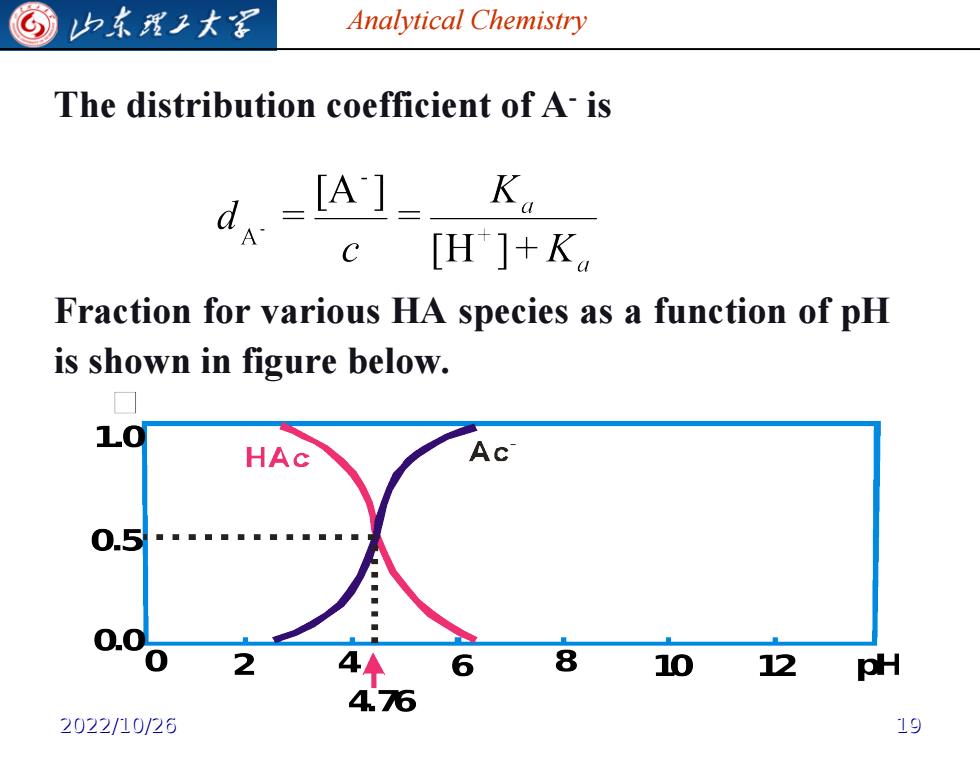
G 归东理工大图 Analytical Chemistry The distribution coefficient of A-is d. [A]_ K。 A c H]+K Fraction for various HA species as a function of pH is shown in figure below. 10 HAc AC 0.5 12 pH 2022/10/26 19
Analytical Chemistry The distribution coefficient of A- is Fraction for various HA species as a function of pH is shown in figure below. 2022/10/26 19