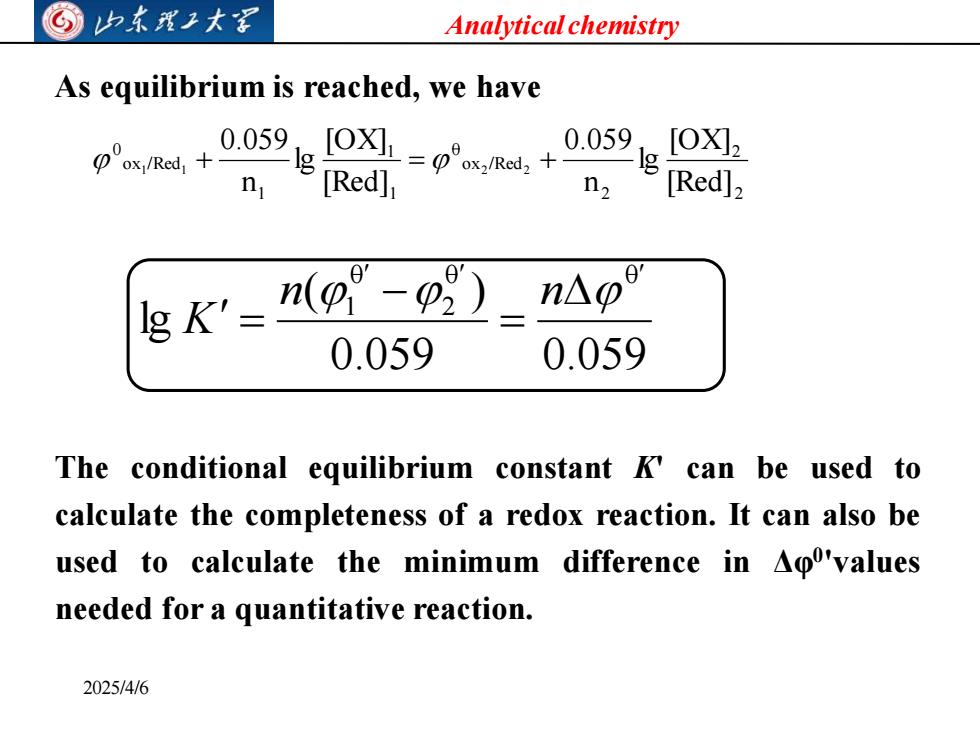
归东我工大写 Analytical chemistry As equilibrium is reached,we have p'x/Ra +0059gIO=pm.+0059e n [Red] n, Red]2 g k'=n(p 1-02) n△o 0.059 0.059 The conditional equilibrium constant K'can be used to calculate the completeness of a redox reaction.It can also be used to calculate the minimum difference in Ao'values needed for a quantitative reaction. 2025/4/6
Analytical chemistry 2025/4/6 As equilibrium is reached, we have 2 2 2 o x /Red 0 1 1 1 o x /Red 0 [Red] [OX] lg n 0.059 [Red] [OX] lg n 0.059 1 1 + = 2 2 + 0.059 0.059 ( ) lg 0 0 2 0 1 = − = n n K The conditional equilibrium constant K' can be used to calculate the completeness of a redox reaction. It can also be used to calculate the minimum difference in Δφ0 'values needed for a quantitative reaction

山东我工大 Analytical chemistry n2OX+n Red2 =n2Red +n OX2 [Redh≥10,02≥10 at sp,Ox [Red]2 e=85-n 0.059 if nj=n2=1 ΛE9≥0.059×6=0.35V If nj=n2-2 4E≥0.059x6 =0.18V 2 2025/4/6
Analytical chemistry 2025/4/6 n2 OX1 + n1 Red2 = n2 Red1 + n1 OX2 3 2 3 2 1 1 10 [Red] [OX] 10 [Ox] [Red] at sp , , 3(n n ) [R ] [O ] [O ] [R ] lg 0.059 n n ' lg ' n 1 2 2 n 2 n 1 n 1 0 1 2 1 1 2 2 = + = E K if n1=n2=1 0.059 6 0.35V 0 = E If n1=n2=2 0.18V 2 0 0.059 6 = E
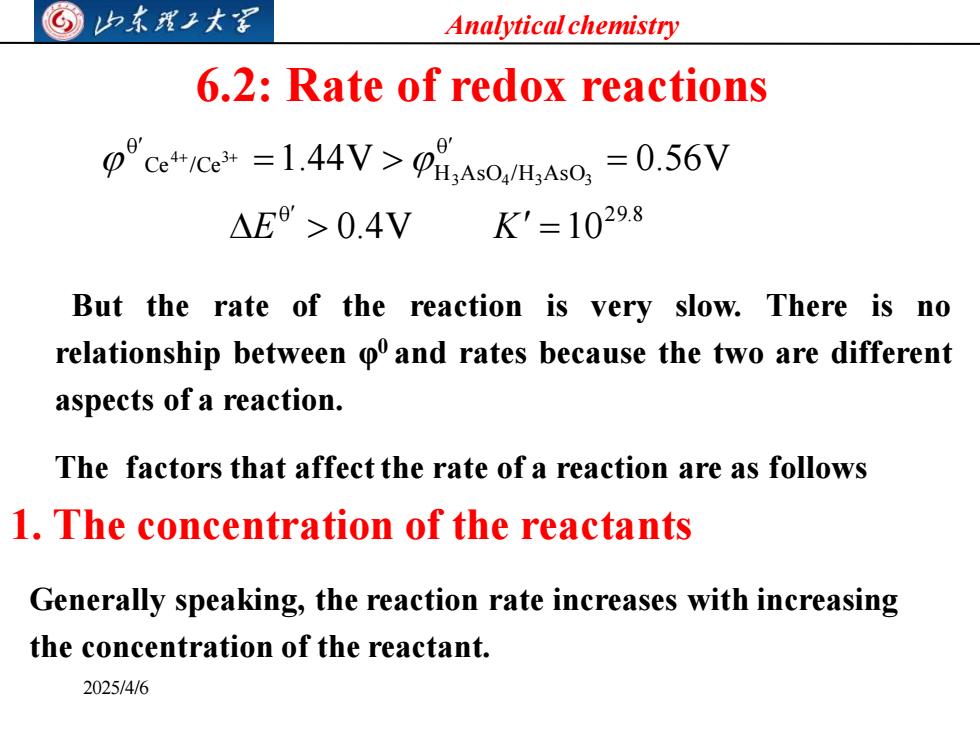
Analytical chemistry 6.2:Rate of redox reactions cC=1.44V=0.56V △E>0.4V K'=10298 But the rate of the reaction is very slow.There is no relationship between o and rates because the two are different aspects of a reaction. The factors that affect the rate of a reaction are as follows 1.The concentration of the reactants Generally speaking,the reaction rate increases with increasing the concentration of the reactant. 2025/4/6
Analytical chemistry 2025/4/6 6.2: Rate of redox reactions But the rate of the reaction is very slow. There is no relationship between φ 0 and rates because the two are different aspects of a reaction. 0 2 9.8 0 Ce /Ce H AsO / H AsO 0 0.4V 10 1.44V 0.56V 3 4 3 3 4 3 = = = + + E K 1. The concentration of the reactants The factors that affect the rate of a reaction are as follows Generally speaking, the reaction rate increases with increasing the concentration of the reactant