
0山东龙工大 Analytical chemistry Fe*+H,OFe(OH)]2++H* Fe*+CI [FeCl]2+ Therefore,the total concentration of Fe()in the solution may be expressed as c(Fe3)=Fe3*+Fe(OH]2++[FeCI]2++. [Fe3+] Where a is the side reaction coefficient of Fe3+,similarly,to the Fe2+ 2025/4/6
Analytical chemistry 2025/4/6 Fe Cl [FeCl] Fe H O [Fe(OH)] H 3 2 2 2 3 + − + + + + + + + c(Fe3+ ) = Fe3+ +[Fe(OH)]2+ +[FeCl]2+ + Therefore, the total concentration of Fe(Ⅲ) in the solution may be expressed as + + = + 3 3 3 Fe Fe [Fe ] c Where α is the side reaction coefficient of Fe3+ , similarly, to the Fe2+
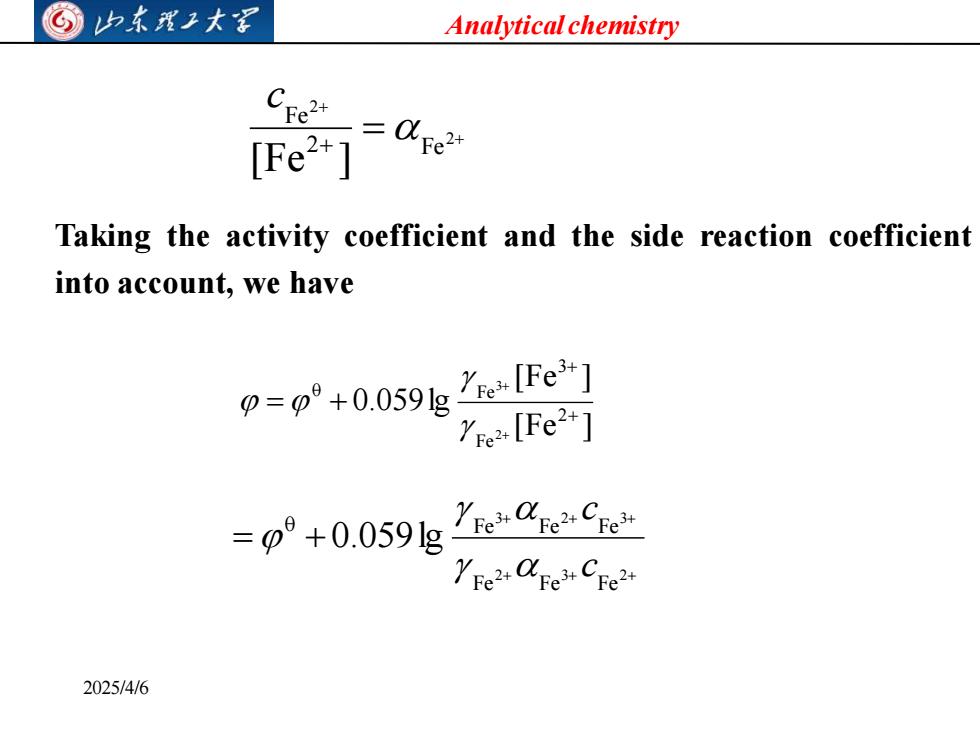
©山东我卫大罩 Analytical chemistry [Fe2+] Taking the activity coefficient and the side reaction coefficient into account,we have 0=p°+0.059g"elFe3] ye2+[Fe2+] =0°+0.059g1e8e9e 2025/4/6
Analytical chemistry 2025/4/6 + + = + 2 2 2 Fe Fe [Fe ] c [Fe ] [Fe ] 0.059lg 2 Fe 3 0 Fe 2 3 + + + + = + Taking the activity coefficient and the side reaction coefficient into account, we have + + + + + + = + 2 3 2 3 2 3 Fe Fe Fe 0 Fe Fe Fe 0.059lg c c

G山东茂子大图 Analytical chemistry When c(Fe3+)=c(fe2+)=1 mol/L The equation may be expressed as p=p°+0.0591g =0 Where oo'is called the conditional potential Obviously,the conditional potential of a couple is just the potential that is measured when the total concentration of both the oxidized and reduced forms are equal to 1 mol/L. Some factors may affect the conditional potential. 2025/4/6
Analytical chemistry 2025/4/6 When c(Fe3+ )=c(fe2+ )=1 mol/L The equation may be expressed as 0.059lg ' 0 Fe Fe 0 Fe Fe 2 3 3 2 = + = + + + + Where φ 0 ' is called the conditional potential Obviously, the conditional potential of a couple is just the potential that is measured when the total concentration of both the oxidized and reduced forms are equal to 1 mol/L. Some factors may affect the conditional potential
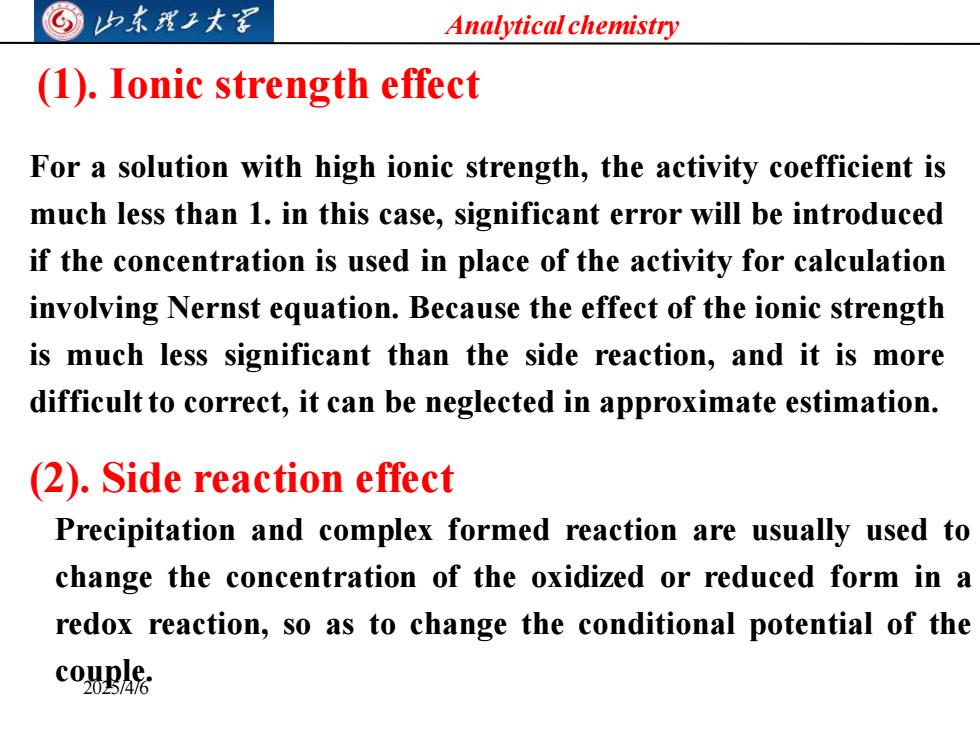
G归东我工大军 Analytical chemistry (1).Ionic strength effect For a solution with high ionic strength,the activity coefficient is much less than 1.in this case,significant error will be introduced if the concentration is used in place of the activity for calculation involving Nernst equation.Because the effect of the ionic strength is much less significant than the side reaction,and it is more difficult to correct,it can be neglected in approximate estimation. (2).Side reaction effect Precipitation and complex formed reaction are usually used to change the concentration of the oxidized or reduced form in a redox reaction,so as to change the conditional potential of the couBA
Analytical chemistry 2025/4/6 (1). Ionic strength effect For a solution with high ionic strength, the activity coefficient is much less than 1. in this case, significant error will be introduced if the concentration is used in place of the activity for calculation involving Nernst equation. Because the effect of the ionic strength is much less significant than the side reaction, and it is more difficultto correct, it can be neglected in approximate estimation. (2). Side reaction effect Precipitation and complex formed reaction are usually used to change the concentration of the oxidized or reduced form in a redox reaction, so as to change the conditional potential of the couple

归东理大 Analytical chemistry When a precipitating agent that can precipitate the oxidized form is added,the conditional potential of the couple decreases.If the reduced form precipitates,however,the conditional potential will increase. 2.Equilibrium constant The equilibrium constant indicates the ability of a redox reaction to proceed.If the conditional potential is employed,then we obtain a conditional equilibrium constant. n2OX +n Red2 =n2Red+n OX2 2025/4/6
Analytical chemistry 2025/4/6 When a precipitating agent that can precipitate the oxidized form is added, the conditional potential of the couple decreases. If the reduced form precipitates, however, the conditional potential will increase. 2. Equilibrium constant The equilibrium constant indicates the ability of a redox reaction to proceed. If the conditional potential is employed, then we obtain a conditional equilibrium constant. n2 OX1 + n1 Red2 = n2 Red1 + n1 OX2