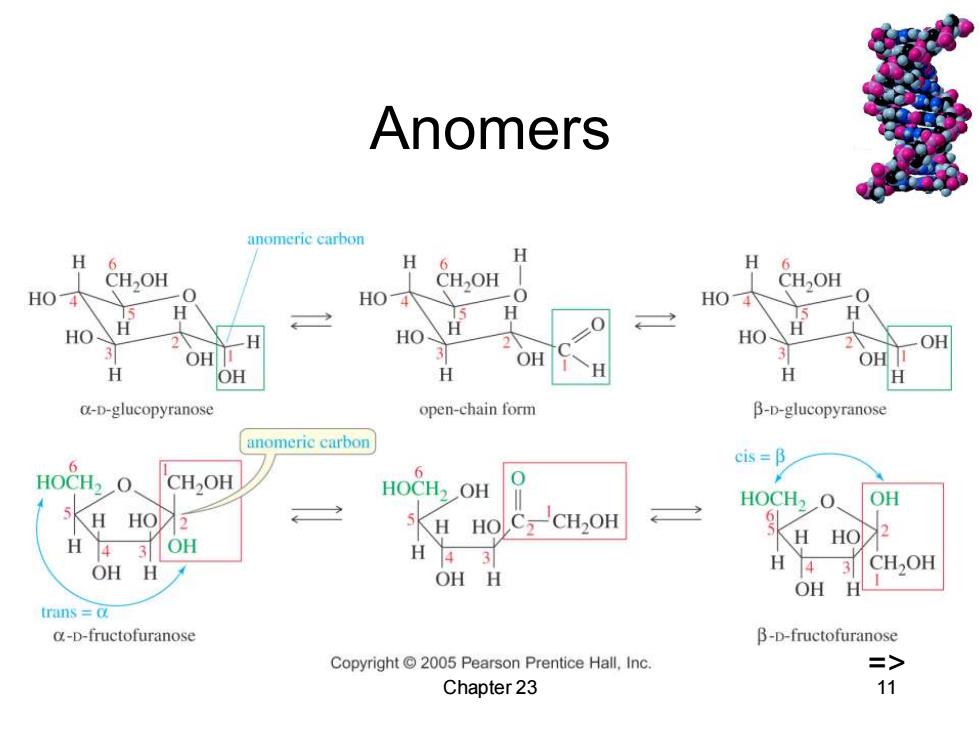
Anomers anomeric carbon H H 6 H H CH,OH OH CH,OH H04 H04 0 HO4 个 H H 》 HO H HO- HO OH OH OH 3 OH H OH H H H a-D-glucopyranose open-chain form B-D-glucopyranose anomeric carbon cis =B HOCH2O CH,OH 6 HOCH2OH HOCH2O. OH HO 2 5KH OH HO2 H 43 OH H 3 CH2OH OH H OH H trans c o-D-fructofuranose B-D-fructofuranose Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 23 11
Chapter 23 11 Anomers =>
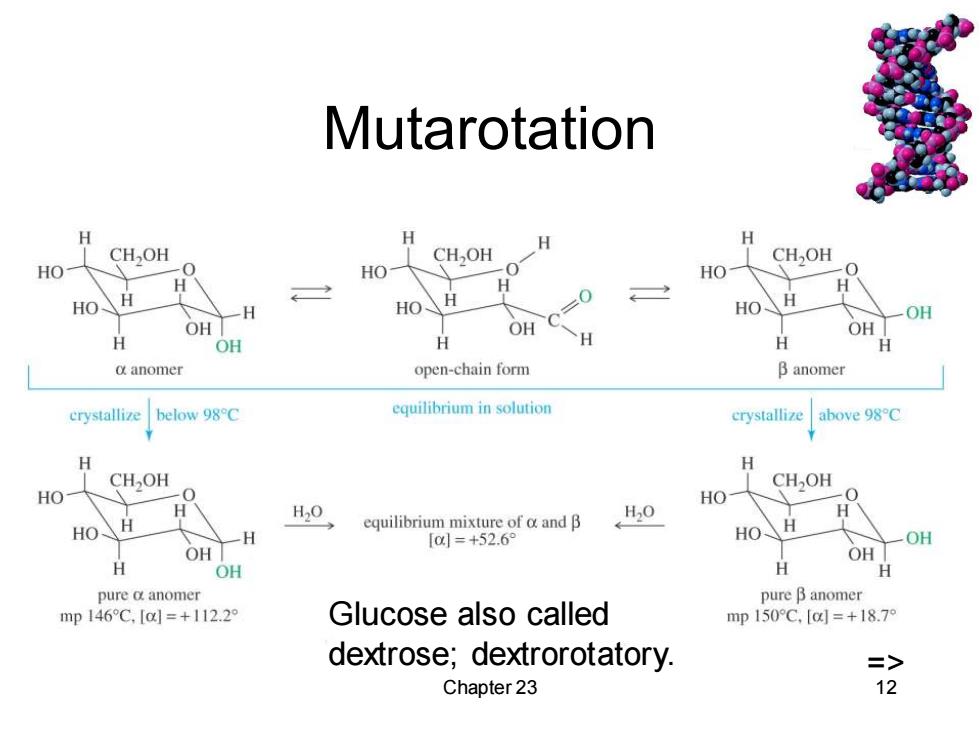
Mutarotation H 夕 CH,OH CHOH CH,OH HO -0 HO HO H H HO H H HO HO、 OH OH OH OH H OH y H H anomer open-chain form B anomer crystallize below 98C equilibrium in solution crystallize above 98C H CHOH CHOH HO HO H H20 equilibrium mixture of o and B H20 H HO H [01=+52.69 HO OH OH OH H OH H H pure a anomer pure B anomer mp146C,[a=+112.29 Glucose also called mp150°C,{c=+18.7 dextrose;dextrorotatory. => Chapter 23 12
Chapter 23 12 Mutarotation => Glucose also called dextrose; dextrorotatory
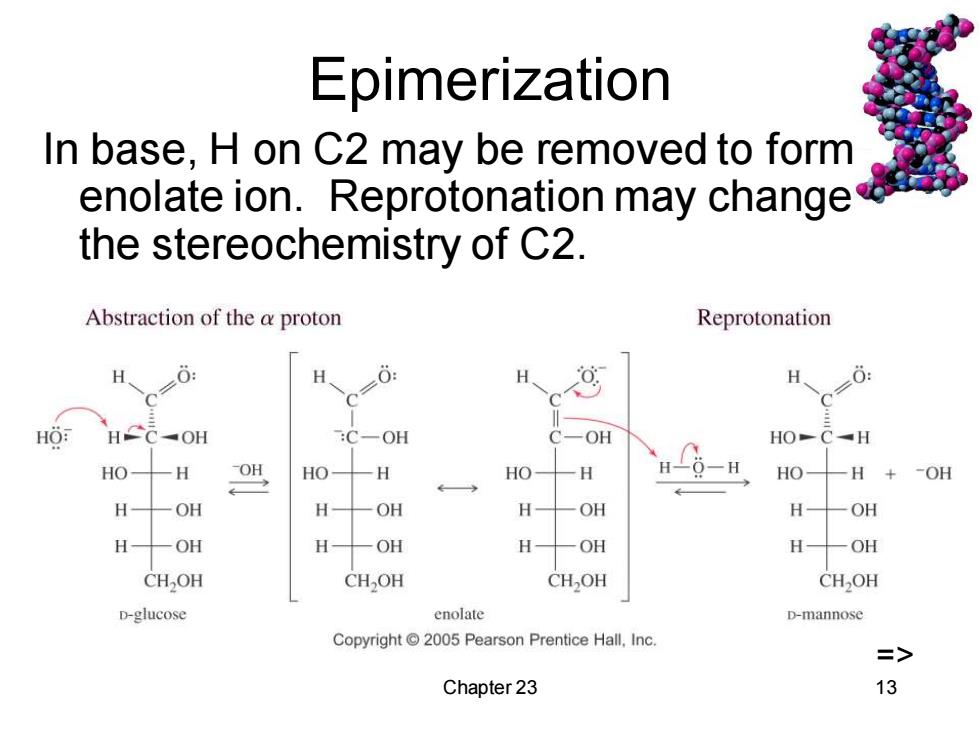
Epimerization In base,H on C2 may be removed to form enolate ion.Reprotonation may change the stereochemistry of C2. Abstraction of the a proton Reprotonation H H、 HO HCC-OH C-OH OH HO-C-H HO H OH HO H HO H HO- -H+ -OH ←→ H OH H OH H OH H OH H OH H OH H- OH H 一OH CH2OH CH>OH CHOH CHOH D-glucose enolate D-mannose Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 23 13
Chapter 23 13 Epimerization In base, H on C2 may be removed to form enolate ion. Reprotonation may change the stereochemistry of C2. =>
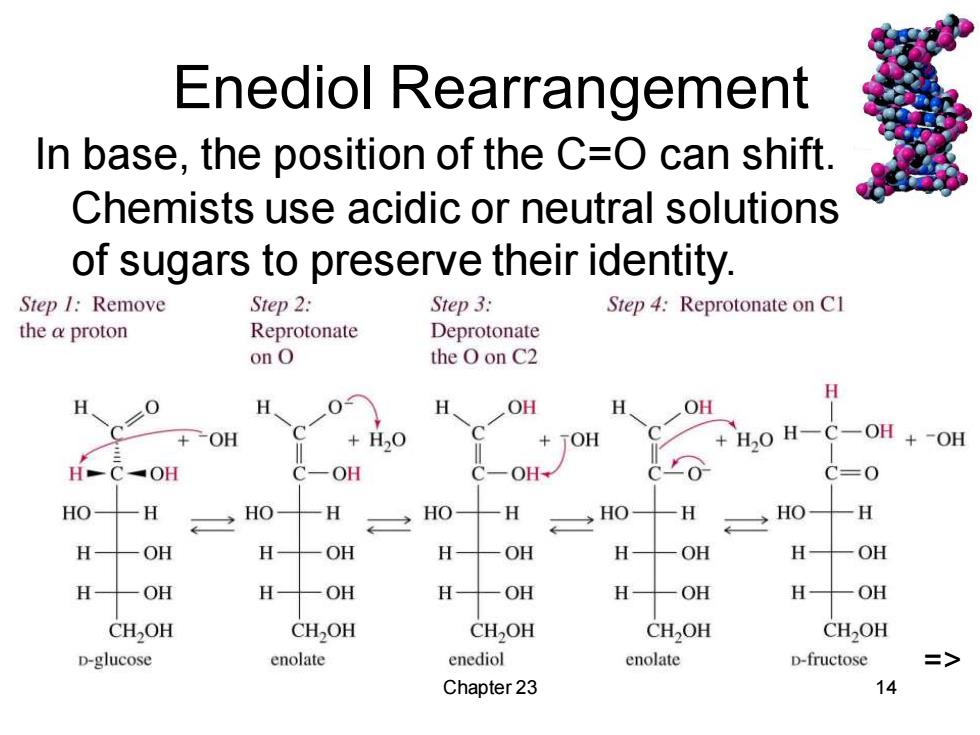
Enediol Rearrangement In base,the position of the C=O can shift Chemists use acidic or neutral solutions of sugars to preserve their identity. Step I:Remove Step 2: Step 3: Step 4:Reprotonate on CI the a proton Reprotonate Deprotonate onO the O on C2 H H H OH OH +OH H,O TOH H20 H-C- OH +-OH H-C-OH OH OH+ C=0 HO H HO H HO- 一H →HO H HO 一H H OH H -OH H- OH H OH H OH H一OH H OH H一OH H OH H -OH CH,OH CHOH CH,OH CH2OH CH2OH D-glucose enolate enediol enolate D-fructose => Chapter 23 14
Chapter 23 14 Enediol Rearrangement In base, the position of the C=O can shift. Chemists use acidic or neutral solutions of sugars to preserve their identity. =>
Reduction of Simple Sugars C=O of aldoses or ketoses can be reduced to C-OH by NaBHa or H2/Ni. Name the sugar alcohol by adding -itol to the root name of the sugar. Reduction of D-glucose produces D-glucitol,commonly called D-sorbitol. Reduction of D-fructose produces a mixture of D-glucitol and D-mannitol. => Chapter 23 15
Chapter 23 15 Reduction of Simple Sugars • C=O of aldoses or ketoses can be reduced to C-OH by NaBH4 or H2 /Ni. • Name the sugar alcohol by adding -itol to the root name of the sugar. • Reduction of D-glucose produces D-glucitol, commonly called D-sorbitol. • Reduction of D-fructose produces a mixture of D-glucitol and D-mannitol. =>