
Physical Properties Melting points: -More symmetrical than corresponding alkane,pack better into crystals,so higher MP. Boiling points: Dependent on dipole moment,so ortho meta para,for disubstituted benzenes. Density: -More dense than nonaromatics,less dense than water. Solubility: -Generally insoluble in water
Physical Properties ❖Melting points: ◼More symmetrical than corresponding alkane, pack better into crystals, so higher MP. ❖Boiling points: ◼ Dependent on dipole moment, so ortho > meta > para, for disubstituted benzenes. ❖Density: ◼More dense than nonaromatics, less dense than water. ❖Solubility: ◼Generally insoluble in water

Physical Properties Physical Properties of benzene 1.It is a colourless liquid with boiling point 80C. 2.It is insoluble in water but soluble in organic solvent. 3.It burns with a sooty flame and it is highly inflammable. 4.It is carcinogenic..致癌物(质)的
Physical Properties Physical Properties of benzene 1. It is a colourless liquid with boiling point 80oC. 2. It is insoluble in water but soluble in organic solvent. 3. It burns with a sooty flame and it is highly inflammable. 4. It is carcinogenic.致癌物(质)的
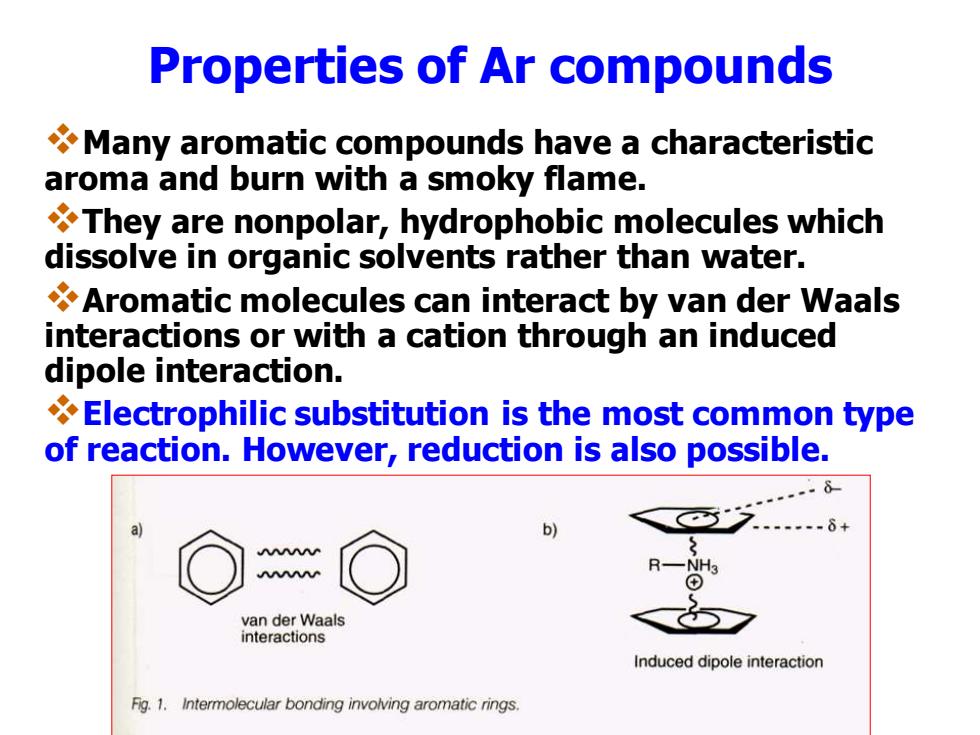
Properties of Ar compounds Many aromatic compounds have a characteristic aroma and burn with a smoky flame. They are nonpolar,hydrophobic molecules which dissolve in organic solvents rather than water. Aromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. Electrophilic substitution is the most common type of reaction.However,reduction is also possible. b) van der Waals interactions Induced dipole interaction Fig.1.Intermolecular bonding involving aromatic rings
Properties of Ar compounds ❖Many aromatic compounds have a characteristic aroma and burn with a smoky flame. ❖They are nonpolar, hydrophobic molecules which dissolve in organic solvents rather than water. ❖Aromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. ❖Electrophilic substitution is the most common type of reaction. However, reduction is also possible

Sec 2 Aromaticity and Reactivity Reactivity反应性 不易加成(苯环骨架不断开) 发生取代(亲电取代ES) 侧链反应 Aromaticity芳香性化合物特点 大的不饱和度(①≥4)Ω=? (环状)平面分子 ■易进行亲电取代反应 芳香性
Sec 2 Aromaticity and Reactivity ❖Reactivity反应性 ◼不易加成(苯环骨架不断开) ◼发生取代(亲电取代 E.S) ◼侧链反应 ❖Aromaticity芳香性化合物特点 ◼大的不饱和度(≧4) =? ◼(环状)平面分子 ◼易进行亲电取代反应 芳香性
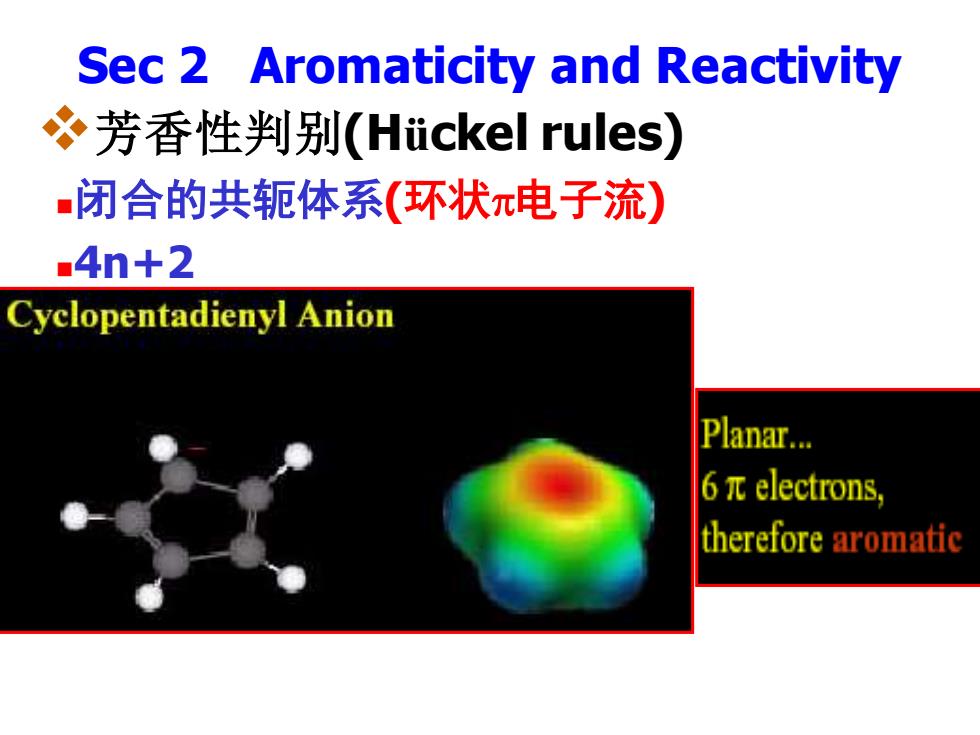
Sec 2 Aromaticity and Reactivity 芳香性判别(Hiickel rules) 闭合的共轭体系(环状π电子流) .4n+2 Cyclopentadienyl Anion Planar... 6πelectrons, therefore aromatic
Sec 2 Aromaticity and Reactivity ❖芳香性判别(Hückel rules) ◼闭合的共轭体系(环状电子流) ◼4n+2