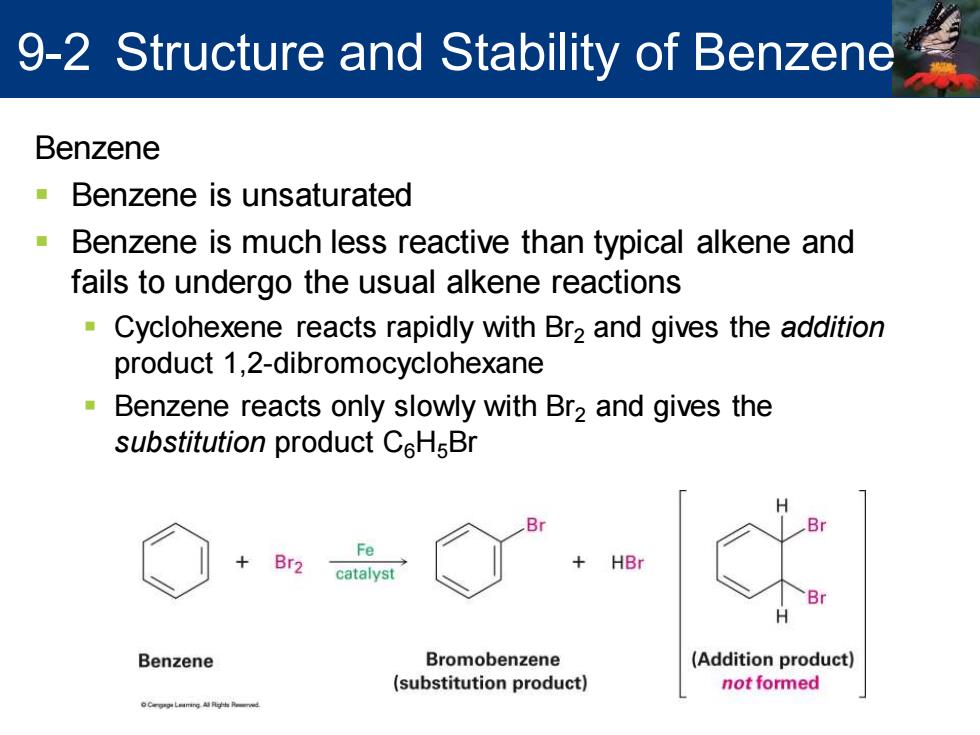
9-2 Structure and Stability of Benzene Benzene -Benzene is unsaturated ■ Benzene is much less reactive than typical alkene and fails to undergo the usual alkene reactions Cyclohexene reacts rapidly with Br2 and gives the addition product 1,2-dibromocyclohexane Benzene reacts only slowly with Br2 and gives the substitution product CeHsBr Fe Br2 catalyst HBr Br Benzene Bromobenzene (Addition product) (substitution product) not formed
Benzene ▪ Benzene is unsaturated ▪ Benzene is much less reactive than typical alkene and fails to undergo the usual alkene reactions ▪ Cyclohexene reacts rapidly with Br2 and gives the addition product 1,2-dibromocyclohexane ▪ Benzene reacts only slowly with Br2 and gives the substitution product C6H5Br 9-2 Structure and Stability of Benzene

Structure and Stability of Benzene A quantitative idea of benzene's stability is obtained from heats of hydrogenation Benzene is 150 kJ/mol (36 kcal/mol)more stable than might be expected for“cyclohexatriene” Benzene 150 kJ/mol (difference】 Cyclohexa-1,3-diene -356 kJ/mol (expected) Cyclohexene -230 kJ/mol -206 kJ/mol (actual) -118 kJ/mol Cyclohexane
A quantitative idea of benzene’s stability is obtained from heats of hydrogenation ▪ Benzene is 150 kJ/mol (36 kcal/mol) more stable than might be expected for “cyclohexatriene” Structure and Stability of Benzene
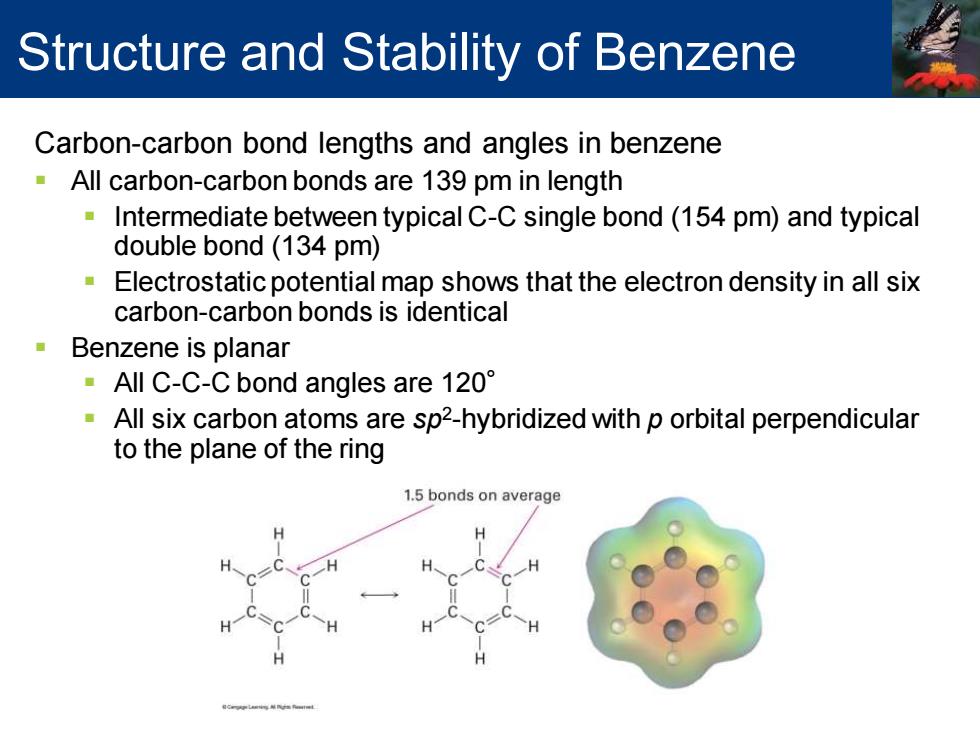
Structure and Stability of Benzene Carbon-carbon bond lengths and angles in benzene All carbon-carbon bonds are 139 pm in length Intermediate between typical C-C single bond(154 pm)and typical double bond (134 pm) Electrostatic potential map shows that the electron density in all six carbon-carbon bonds is identical ■ Benzene is planar ·AllC-C-C bond angles are120° All six carbon atoms are sp2-hybridized with p orbital perpendicular to the plane of the ring 1.5 bonds on average
Carbon-carbon bond lengths and angles in benzene ▪ All carbon-carbon bonds are 139 pm in length ▪ Intermediate between typical C-C single bond (154 pm) and typical double bond (134 pm) ▪ Electrostatic potential map shows that the electron density in all six carbon-carbon bonds is identical ▪ Benzene is planar ▪ All C-C-C bond angles are 120° ▪ All six carbon atoms are sp2 -hybridized with p orbital perpendicular to the plane of the ring Structure and Stability of Benzene
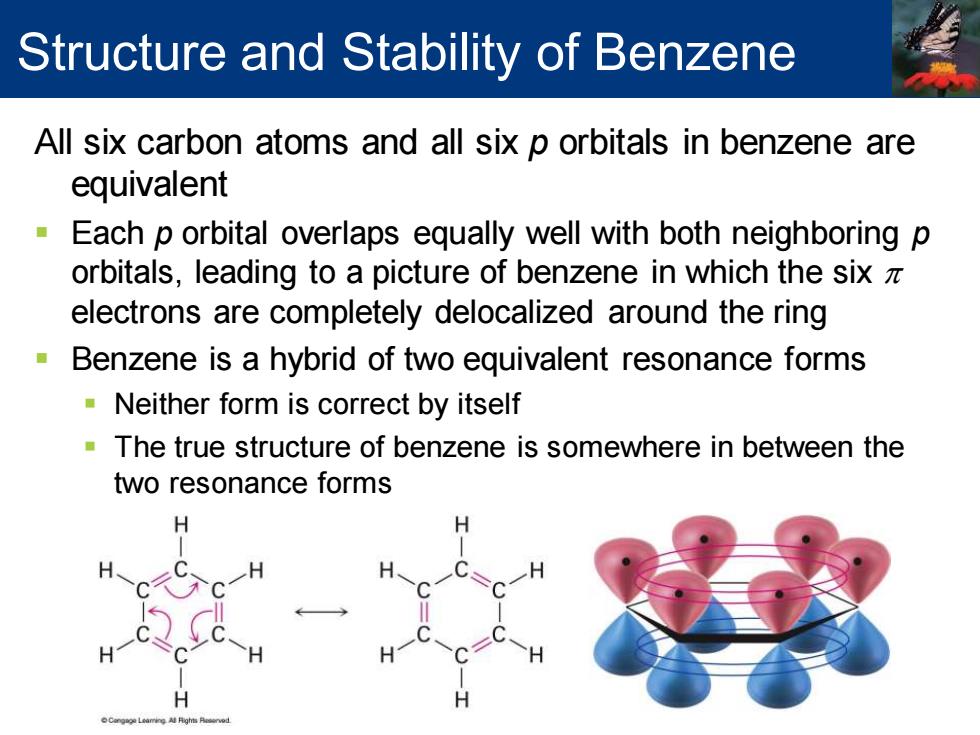
Structure and Stability of Benzene All six carbon atoms and all six p orbitals in benzene are equivalent Each p orbital overlaps equally well with both neighboring p orbitals,leading to a picture of benzene in which the sixz electrons are completely delocalized around the ring Benzene is a hybrid of two equivalent resonance forms Neither form is correct by itself The true structure of benzene is somewhere in between the two resonance forms
All six carbon atoms and all six p orbitals in benzene are equivalent ▪ Each p orbital overlaps equally well with both neighboring p orbitals, leading to a picture of benzene in which the six p electrons are completely delocalized around the ring ▪ Benzene is a hybrid of two equivalent resonance forms ▪ Neither form is correct by itself ▪ The true structure of benzene is somewhere in between the two resonance forms Structure and Stability of Benzene

Structure and Stability of Benzene Six p atomic orbitals combine in a cyclic manner,six benzenez molecular orbitals result The three lower-energy molecular orbitals,denoted 2,and V3,are bonding combinations 12and ya have the same energy and are said to be degenerate Antibonding 路 +++++十 Six p atomic orbitals 双的科 Bonding Six benzene molecular orbitals
Six p atomic orbitals combine in a cyclic manner, six benzene p molecular orbitals result ▪ The three lower-energy molecular orbitals, denoted y1 , y2 ,and y3 ,are bonding combinations ▪ y2 and y3 have the same energy and are said to be degenerate Structure and Stability of Benzene