
Three resonance forms can be written for formic acid. 0 Q 0- H-C-O-H →H-C0H H-CtO-H major minor very minor The second structure requires the C-O-H bonds to be co-planar. One of the unshared lone pairs of oxygen is delocalized into the electrophilic system of the carbonyl group (One of the lone pairs on the hydroxyl oxygen is conjugated with the C=O double bond). Acidity Carboxylic acids can dissociate in aqueous solution into carboxylate ions and protons The equilibrium constant for this process is Ka,and more frequently we talk in terms of pK O-H+H,OR -0-+H,0 K= [R一CO2]H,O [R-CO,H] pK -l0g10 K Ch20 Carboxylic Acids (landscape) Page 6
Ch20 Carboxylic Acids (landscape) Page 6 Three resonance forms can be written for formic acid. The second structure requires the C-O-H bonds to be co-planar. One of the unshared lone pairs of oxygen is delocalized into the electrophilic system of the carbonyl group. (One of the lone pairs on the hydroxyl oxygen is conjugated with the C=O double bond). Acidity Carboxylic acids can dissociate in aqueous solution into carboxylate ions and protons. The equilibrium constant for this process is Ka , and more frequently we talk in terms of pKa . H C O O H H C O O- H H C O O- H + + major minor very minor
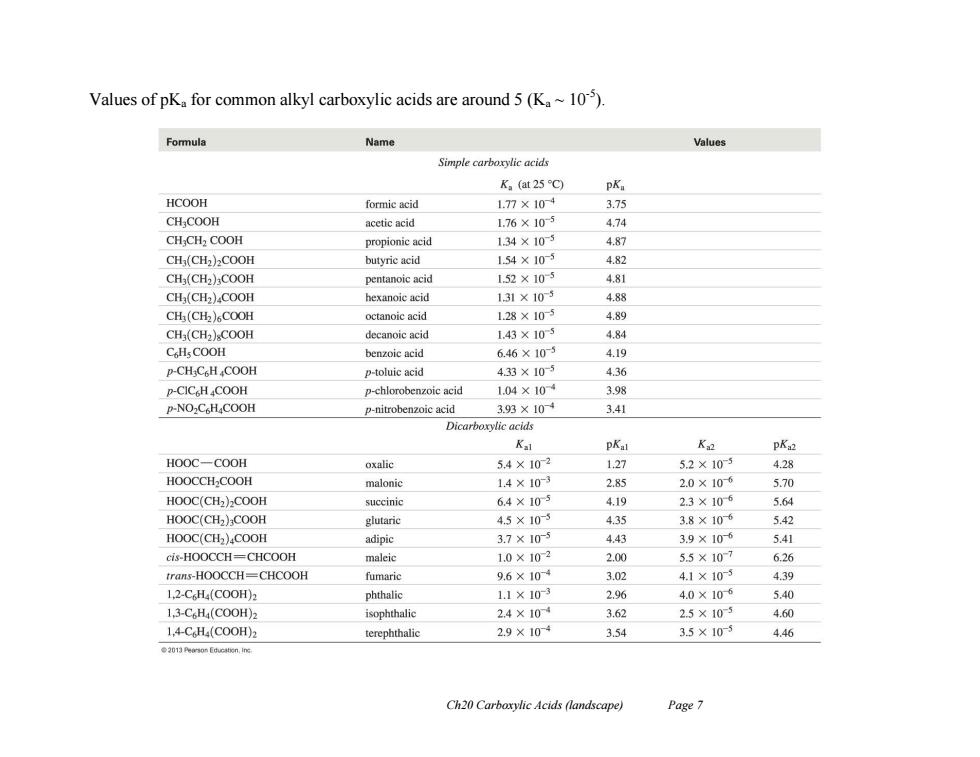
Values of pK for common alkyl carboxylic acids are around 5(K10). Formula Name Values Simple carboxylic acids K。(at25C) pKa HCOOH formic acid 1.77×104 3.75 CH;COOH acetic acid 1,76×105 474 CH:CH2 COOH propionic acid 1.34×105 4.87 CH(CH2)2COOH butyric acid 154×105 4.82 CH3(CH2)COOH pentanoic acid 1.52×105 4.81 CH3(CH2)4COOH hexanoic acid 131×105 4.88 CH (CH2)COOH octanoic acid 1.28×105 4.89 CH(CH2)COOH decanoic acid 1.43×105 4.84 CHsCOOH benzoic acid 6.46×10 4.19 P-CH;CsH COOH p-toluic acid 433×105 4.36 p-CIC HCOOH p-chlorobenzoic acid 1.04×104 3.98 P-NO2C H4COOH p-nitrobenzoic acid 3.93×104 3.41 Dicarboxylic acids Kal pKal K pK32 HOOC一COOH oxalic 5.4×102 1.27 5.2×105 4.28 HOOCCH2COOH malonic 1.4×103 2.85 2.0×106 5.70 HOOC(CH2)COOH succinic 6.4×105 4.19 2.3×106 5.64 HOOC(CH2)COOH glutaric 4.5×105 4.35 3.8×106 5.42 HOOC(CH2)COOH adipic 3.7×105 4.43 3.9×106 5.41 cis-HOOCCH=CHCOOH maleic 1.0×102 2.00 55×107 6.26 trans-HOOCCH=CHCOOH fumaric 9.6×104 3.02 4.1×103 4.39 1,2-C6H(C0OH)2 phthalic 1.1×103 2.96 4.0×106 540 1,3-C6H(C00H)2 isophthalie 2.4×10 3.62 2.5×105 4.60 1,4-CH(C00H)2 terephthalic 2.9×10 3.54 3.5×105 4.46 Ch20 Carboxylic Acids (landscape) Page 7
Ch20 Carboxylic Acids (landscape) Page 7 Values of pKa for common alkyl carboxylic acids are around 5 (Ka ~ 10-5 )

E.g.ethanoic acid has pK=4.74,(alcohols have pK~16,so carboxylic acids are about 10 times more acidic than alcohols). The reason why carboxylic acids are much more acidic than alcohols is because the carboxylate anion is much more stable than the alkoxide anion. Both alcohols and carboxylic acids are acidic since their respective O-H bonds can be broken heterolytically, giving a proton and an oxygen anion. R一0-H+H,δ:÷ R-6: +H,0冰6 (K,=10-16 alcohol alkoxide K5 R -C-0-H+H,ǒ:。→ R →R H.O+ (K≡105 acid carboxylate R-0- +HO+ R-OH +H2O 0 R-C HO+ R-COOH H2O stabilization carboxylate The difference lies in the fact that the carboxylate anion has the negative charge spread out over two oxygen atoms, whereas the alcohol has the negative charge localized on a single oxygen atom. Ch20 Carboxylic Acids (landscape) Page 8
Ch20 Carboxylic Acids (landscape) Page 8 E.g. ethanoic acid has pKa = 4.74, (alcohols have pKa ~ 16, so carboxylic acids are about 1011 times more acidic than alcohols). The reason why carboxylic acids are much more acidic than alcohols is because the carboxylate anion is much more stable than the alkoxide anion. Both alcohols and carboxylic acids are acidic since their respective O-H bonds can be broken heterolytically, giving a proton and an oxygen anion. The difference lies in the fact that the carboxylate anion has the negative charge spread out over two oxygen atoms, whereas the alcohol has the negative charge localized on a single oxygen atom

The carboxylate anion can be viewed as a resonance hybrid of the two anionic structures,or as a conjugated system of three interacting p orbitals containing four electrons(like the allylic anion system). 0 02 The C and two oxygens are all sp'hybridized,and the remaining p orbitals create the nt MO system giving rise to the half t bond between each C and O,and the half negative charge on the end oxygens. Dicarboxylic Acids These have two dissociation constants,since they can lose two protons. HO CH2 OH OH 0 CH, malonic acid KL=1.4×10-3 anion K2=2.0×10-6 dianion +2H20 +H30t+H20 +2HO+ 2013 he K for the first dissociation and K2 for the second dissociation,which generates a dianion. K2 is always less than K(the second carboxyl group is less acidic)since it takes extra energy to overcome the second negative charge being so close to the first negative charge. Ch20 Carboxylic Acids (landscape) Page 9
Ch20 Carboxylic Acids (landscape) Page 9 The carboxylate anion can be viewed as a resonance hybrid of the two anionic structures, or as a conjugated system of three interacting p orbitals containing four electrons (like the allylic anion system). The C and two oxygens are all sp2 hybridized, and the remaining p orbitals create the MO system giving rise to the half bond between each C and O, and the half negative charge on the end oxygens. Dicarboxylic Acids These have two dissociation constants, since they can lose two protons. Ka1 for the first dissociation and Ka2 for the second dissociation, which generates a dianion. Ka2 is always less than Ka1 (the second carboxyl group is less acidic) since it takes extra energy to overcome the second negative charge being so close to the first negative charge
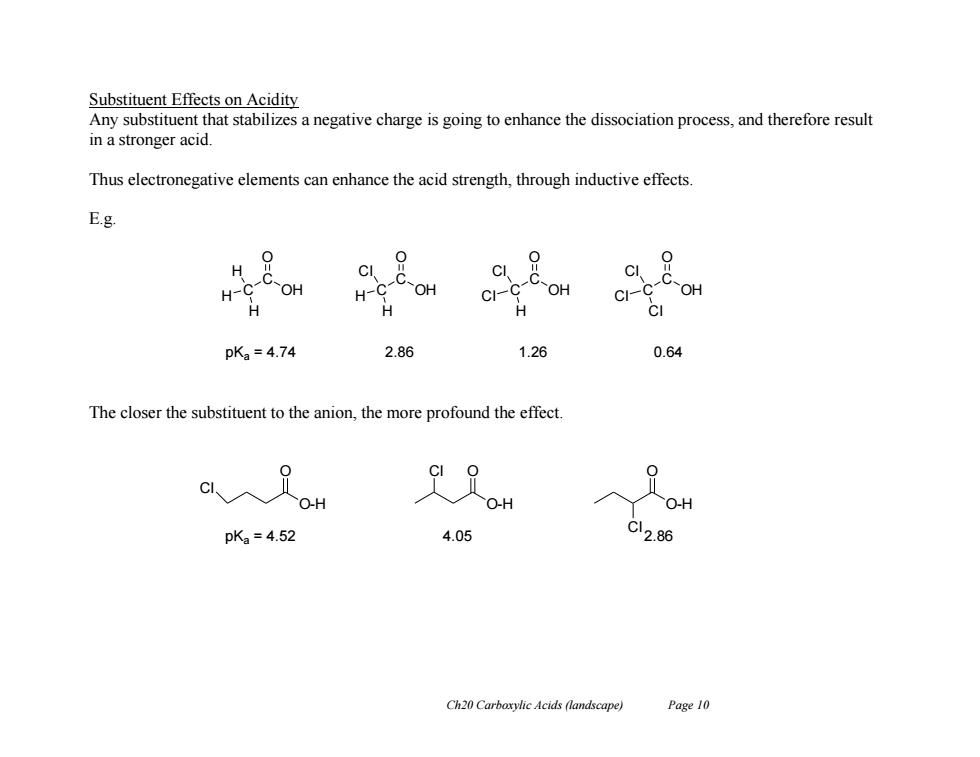
Substituent Effects on Acidity Any substituent that stabilizes a negative charge is going to enhance the dissociation process,and therefore result in a stronger acid. Thus electronegative elements can enhance the acid strength,through inductive effects. E.g. H 9 0 C OH C CI H- H-C OH CI-C OH ci-4 C-OH H H H pKa=4.74 2.86 1.26 0.64 The closer the substituent to the anion,the more profound the effect OH OH O-H pKa=4.52 4.05 C12.86 Ch20 Carboxylic Acids (landscape) Page 10
Ch20 Carboxylic Acids (landscape) Page 10 Substituent Effects on Acidity Any substituent that stabilizes a negative charge is going to enhance the dissociation process, and therefore result in a stronger acid. Thus electronegative elements can enhance the acid strength, through inductive effects. E.g. The closer the substituent to the anion, the more profound the effect. C C OH O H H H C C OH O H Cl H C C OH O Cl Cl H C C OH O Cl Cl Cl pKa = 4.74 2.86 1.26 0.64 O-H O Cl O-H O O-H Cl O Cl pKa = 4.52 4.05 2.86