
Synthetic diamonds:HPHT The majority of commercially available synthetic diamonds are yellow and are produced by so called High Pressure High Temperature(HPHT) 2 mm processes.The yellow color Synthetic diamonds of various colors is caused by nitrogen impurities. grown by the high-pressure high- temperature technique Other colors may also be reproduced such as blue,green or pink 16
The majority of commercially available synthetic diamonds are yellow and are produced by so called High Pressure High Temperature (HPHT) processes. The yellow color is caused by nitrogen impurities. Other colors may also be reproduced such as blue, green or pink Synthetic diamonds: HPHT 16
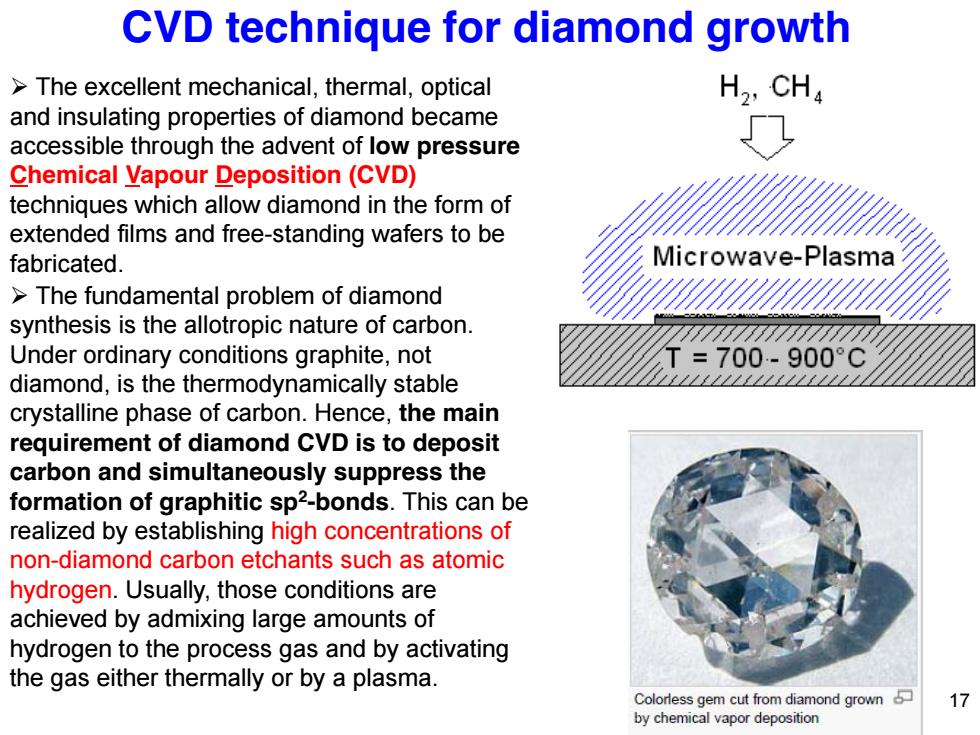
CVD technique for diamond growth >The excellent mechanical,thermal,optical H2,CHa and insulating properties of diamond became accessible through the advent of low pressure Chemical Vapour Deposition(CVD) techniques which allow diamond in the form of extended films and free-standing wafers to be fabricated. licrowave-Plasma The fundamental problem of diamond synthesis is the allotropic nature of carbon. Under ordinary conditions graphite,not T=700-900°C diamond,is the thermodynamically stable ∠∠∠∠∠∠∠∠∠∠∠∠1∠∠∠ crystalline phase of carbon.Hence,the main requirement of diamond CVD is to deposit carbon and simultaneously suppress the formation of graphitic sp2-bonds.This can be realized by establishing high concentrations of non-diamond carbon etchants such as atomic hydrogen.Usually,those conditions are achieved by admixing large amounts of hydrogen to the process gas and by activating the gas either thermally or by a plasma. Colorless gem cut from diamond grown 17 by chemical vapor deposition
¾ The fundamental problem of diamond synthesis is the allotropic nature of carbon. Under ordinary conditions graphite, not diamond, is the thermodynamically stable crystalline phase of carbon. Hence, the main requirement of diamond CVD is to deposit carbon and simultaneously suppress the formation of graphitic sp2-bonds. This can be realized by establishing high concentrations of non-diamond carbon etchants such as atomic hydrogen. Usually, those conditions are achieved by admixing large amounts of hydrogen to the process gas and by activating the gas either thermally or by a plasma. ¾ The excellent mechanical, thermal, optical and insulating properties of diamond became accessible through the advent of low pressure Chemical Vapour Deposition (CVD) techniques which allow diamond in the form of extended films and free-standing wafers to be fabricated. CVD technique for diamond growth 17
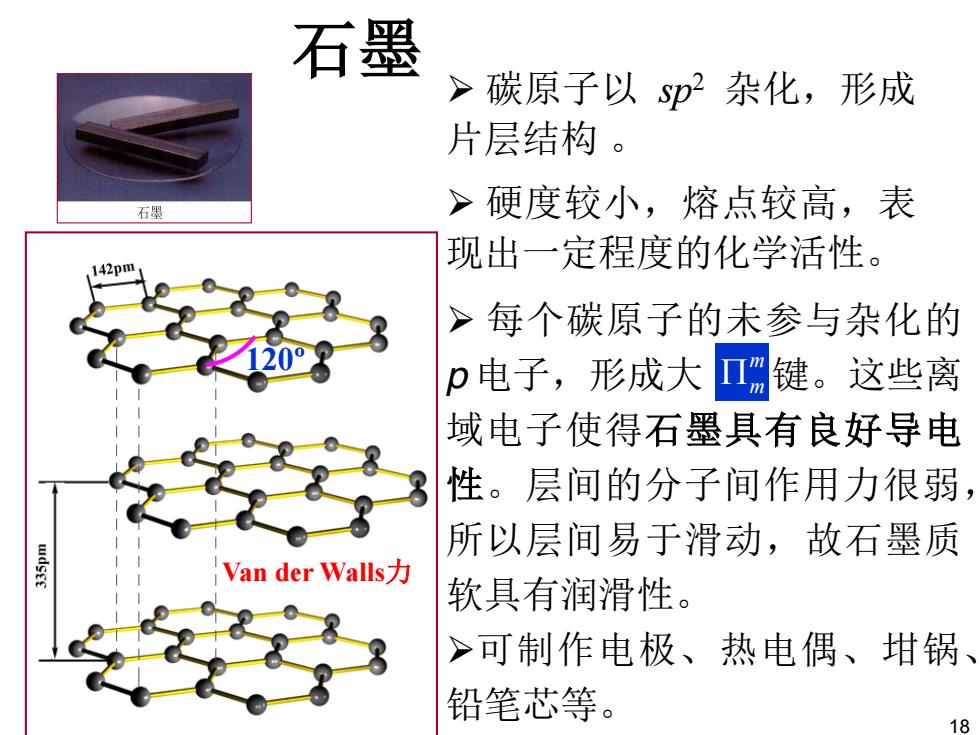
石墨 > 碳原子以sp2杂化,形成 片层结构。 石墨 >硬度较小,熔点较高,表 现出一定程度的化学活性。 42pm >每个碳原子的未参与杂化的 p电子,形成大Π键。这些离 域电子使得石墨具有良好导电 性。层间的分子间作用力很弱, 所以层间易于滑动,故石墨质 Van der Walls,力 软具有润滑性。 >可制作电极、热电偶、坩锅、 铅笔芯等。 18
120º Van der Walls力 石墨 ¾ 每个碳原子的未参与杂化的 p 电子,形成大 键。这些离 域电子使得石墨具有良好导电 性。层间的分子间作用力很弱, 所以层间易于滑动,故石墨质 软具有润滑性。 ¾可制作电极、热电偶、坩锅、 铅笔芯等。 m 3m ¾ 碳原子以 sp2 杂化,形成 片层结构 。 ¾ 硬度较小,熔点较高,表 现出一定程度的化学活性。 18
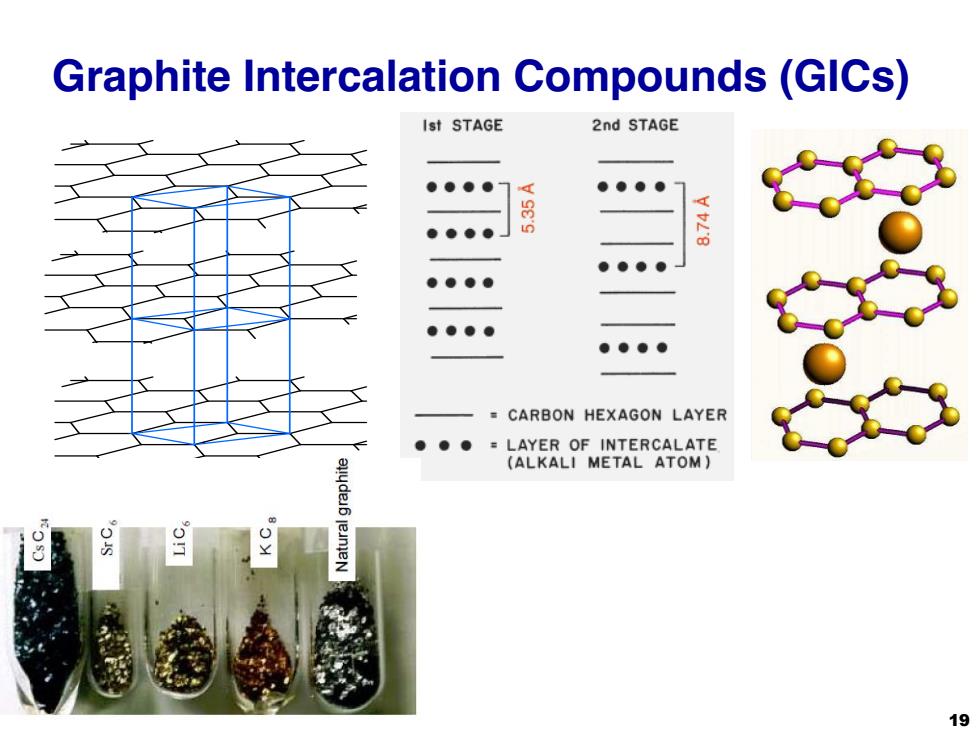
Graphite Intercalation Compounds(GICs) Ist STAGE 2nd STAGE ●●●● ●●●● ●●●● 8 ●●●● ●●●● ●●●● ●●●● CARBON HEXAGON LAYER LAYER OF INTERCALATE (ALKALI METAL ATOM) 19
Graphite Intercalation Compounds (GICs) 19
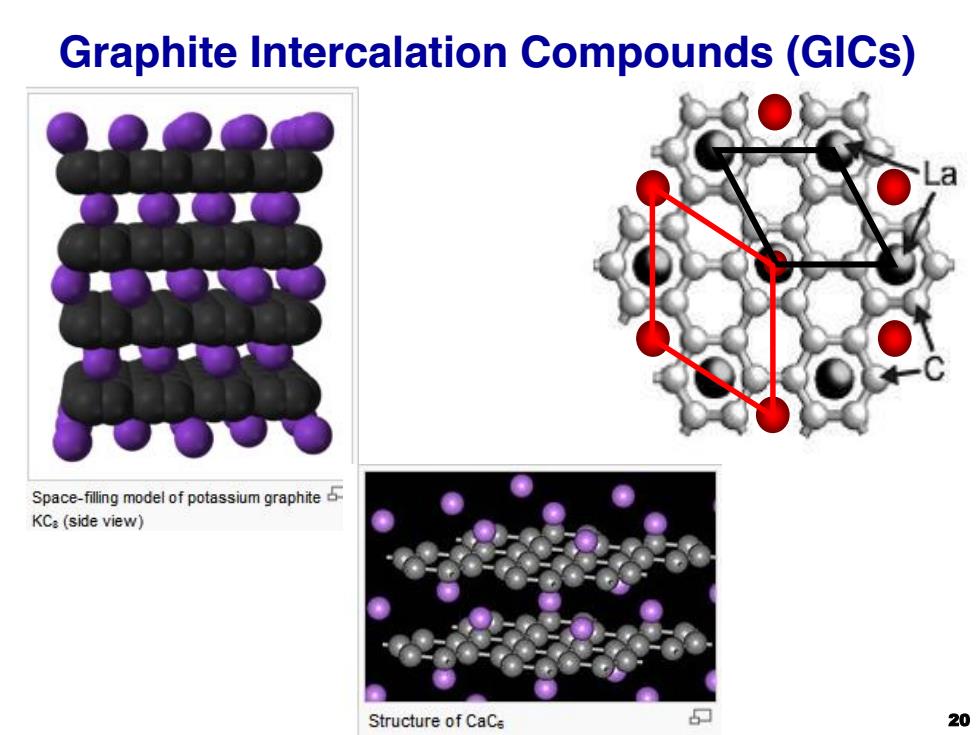
Graphite Intercalation Compounds (GICs) Space-filing model of potassium graphite KC:(side view) Structure of CaCe 20
Graphite Intercalation Compounds (GICs) 20