
The carbon family The most exciting The true but less and amazing side explored side of of carbon carbon Nanotubes Graphite Sheets 1D 2D e The most The common beautiful side of carbon 9 side of 0 carbon Fullerene e Amorphous Carbon OD 3D 排鞋 The shining The dark,soft and hard side of 接移鞋款 接 and tough side carbon of carbon Diamond Graphite 36 3D 11
11

diamond graphite 经学经兴一 、姜立 经经 b C6 7540 70 g h amorphous carbon carbon nanotube 12
diamond graphite C60 C540 C70 amorphous carbon carbon nanotube 12

The system of carbon allotropes spans a range of extremes Synthetic nanocrystalline diamond is the hardest material known.[19] Graphite is one of the softest materials known. Graphite is a very good lubricant,displaying Diamond is the ultimate abrasive superlubricity.[20] Diamond is an excellent electrical insulator.[21] Graphite is a conductor of electricity.(22] Diamond is the best known naturally occurring Some forms of graphite are used for thermal thermal conductor insulation (i.e.firebreaks and heat shields) Diamond is highly transparent. Graphite is opaque Diamond crystallizes in the cubic system. Graphite crystallizes in the hexagonal system.[23] Amorphous carbon is completely isotropic. Carbon nanotubes are among the most anisotropic materials ever produced. ※两个Nobel Prizes! 13
The system of carbon allotropes spans a range of extremes 13 ※ 两个Nobel Prizes!

从“卖炭翁”到“卖纳米碳商” @"孕榜4相分定卖 nano-c 卖炭翁 nanostructured carbon materials that power our world 朝代:唐代 作者:白居易 About Us 原文: News Events 卖炭翁,伐薪烧炭南山中。 。Fullerenes Fullerene Derivatives 满面尘灰烟火色,两鬓苍苍十指黑。 Nanotubes 卖炭得钱何所营?身上衣裳口中食。 Licensing Development Contact Us 可怜身上衣正单,心忧炭贱愿天寒。 http://www.nano-c.com/ 夜来城外一尺雪,晓驾炭车辗冰辙。 牛困人饥日已高,市南门外泥中数。 苏州大德碳纳米科技有限公司 大德科技Suzhou Dade,Carbon,Nanotechnology Co,Ld 通中文■Englian 翩翩两骑来是谁?黄衣使者白衫儿。 站丙关于我日企业售新阅中心产品展示 人才招聘 客户名 所系我们 手把文书口称敕,回车叱牛牵向北。 世界级的富勒烯制备专家 一车炭,千余斤,宫使驱将惜不得。 半匹红绡一丈绫,系向牛头充炭直。 http://www.dadec60.com/
14 从“卖炭翁”到“卖纳米碳商” http://www.nano-c.com/ http://www.dadec60.com/
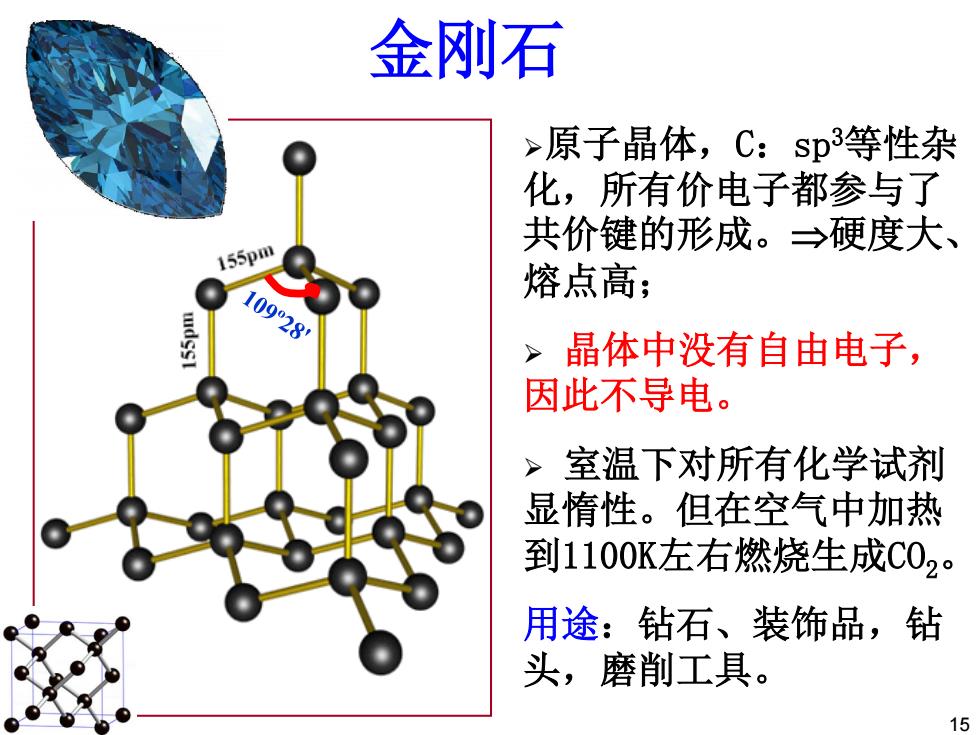
金刚石 >原子晶体,C:sp3等性杂 化,所有价电子都参与了 共价键的形成。→硬度大、 155pm 熔点高; 10928 >晶体中没有自由电子, 因此不导电。 > 室温下对所有化学试剂 显惰性。但在空气中加热 到1100K左右燃烧生成C02。 用途:钻石、装饰品,钻 头,磨削工具。 15
金刚石 ¾原子晶体,C:sp3等性杂 化,所有价电子都参与了 共价键的形成。硬度大、 熔点高; ¾ 晶体中没有自由电子, 因此不导电。 ¾ 室温下对所有化学试剂 显惰性。但在空气中加热 到1100K左右燃烧生成CO2。 用途:钻石、装饰品,钻 头,磨削工具。 15