
河北医科大学药学院 Obstacles needed to be overcome before carbon NMR emerged as a routine tool: Obstacles needed to be overcome before carbon NMR emerged as a routine tool adding to the previous difficuy ical shift ram Chemical Function 三 3 am -1 90-104B 13C Chemical Shift Ranges" Low Fiels Region 核磁共振碳谱例子 Examples of 13C-NMR Spectra For samplos in CDCl solution.The 8 scale is relativo to TMS st 天然药物化学室史清文教授 11
河北医科大学药学院 天然药物化学室史清文教授 11 Obstacles needed to be overcome before carbon NMR emerged as a routine tool : i) As noted, the abundance of 13C in a sample is very low (1.1%), so higher sample concentrations are needed. ii) The 13C nucleus is over fifty times less sensitive than a proton in the NMR experiment, adding to the previous difficulty. . 241 Obstacles needed to be overcome before carbon NMR emerged as a routine tool : iii) Hydrogen atoms bonded to a 13C atom split its NMR signal by 130 to 270 Hz, further complicating the NMR spectrum. iv) Unlike proton NMR spectroscopy, the relative strength of carbon NMR signals are not normally proportional to the number of atoms generating each one. 242 Chemical shift ranges utilized for disfunctionalization of the 13C NMR data Chemical Function Multiplicity Ranges C=O 1 190.0–250.0 CHO 2 190.0–250.0 R–COO–R 1 165.5–190.0 C=C 1 113.0–165.5 2 104.0–167.0 3 100.0–167.0 C–OH 1 61.0–100.0 2 54.0–90.0 3 54.0–90.0 C(OR)2 1 100.0–113.0 CH(OR)2 2 90.0–104.0 243 Low Fiels Region Hight Fiels Region 244 Low Fiels Region Hight Fiels Region 13C Chemical Shift Ranges* * For samples in CDCl3 solution. The d scale is relative to TMS at d = 0. 245 核磁共振碳谱例子 Examples of 13C-NMR Spectra 246

河北医科大学药学院 化学位移:SP,SP,含杂原子 “70" C-0 形进静骑 化学位移:含活泼氢 0- H- DCL-SOH) D EG H H H-H COS HSQC ● C-6is c ald than C-8 but H-s时hanH 天然药物化学室史清文教授 12
河北医科大学药学院 天然药物化学室史清文教授 12 化学位移:SP2, SP3, 含杂原子 O O OCH3 OH HO C=O 247 O O OCH3 OH H3CO OH C=O 248 DMSO OH H-3 H-8 H-6 H-2’ H-3’ H-6 is upfield than H-8 化学位移:含活泼氢 O O OH OH HO 6 3 8 2' 3' 249 A B C D E F G H F(1H,s) H (1H, br.s) D (2H,J=8.0 Hz) G(1H,br.s) A Flavonoid, C15H10O5 Exact Mass: 270.05 E (2H,J=8.0 Hz) O O OH OH HO DMSO 溶剂峰 250 5-OH, 为什么低场?为什么尖锐峰? 4’-OH 和7-OH为什么是个峰包? 1H-1H COSY 251 H-3 H-8 H-6 H-2’ H-6’ H-3’ H-5’ O O OH OH HO 6 3 8 2' 3' HSQC H-8 H-6 H-3 H-3’ H-2’ C-6 C-8 C-3 C-3’ C-2’ C-6 is downfield than C-8, but H-6 is upfield than H-8 O O OH OH HO 6 3 8 2' 3' 252 H-5’ H-6’ C-6’ C-5’
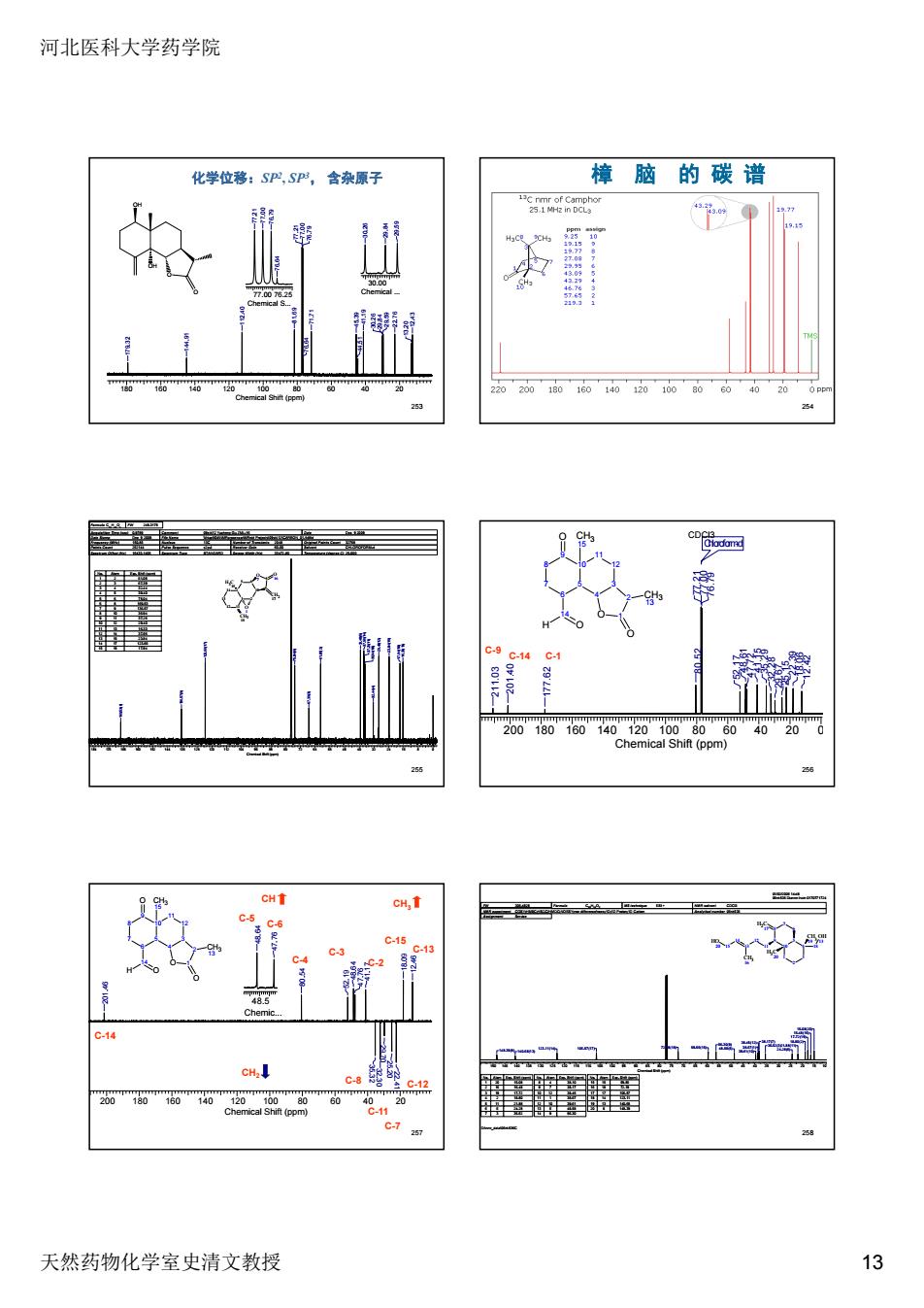
河北医科大学药学院 化学位移:S严,SP,含杂原子 樟脑的碳谱 20200004000000402动 C.t 4020 CH, C3 cH: c 天然药物化学室史清文教授
河北医科大学药学院 天然药物化学室史清文教授 13 O OH OH O 180 160 140 120 100 80 60 40 20 Chemical Shift (ppm) 12.43 13.20 29.59 22.76 30.26 29.84 41.19 44.51 45.39 71.71 76.64 76.79 77.21 77.00 81.69 112.40 144.91 179.32 77.00 76.25 Chemical S... 76.64 77.21 77.00 76.79 30.00 Chemical ... 30.26 29.84 29.59 化学位移:SP2, SP3, 含杂原子 253 樟 脑 的 碳 谱 254 Formula C15H20O3 FW 248.3175 Acquisition Time (sec) 0.9789 Comment 09st412 Yucheng Gu ZML-16 Date Dec 9 2009 Date Stamp Dec 9 2009 File Name \\friapfil04\NMRprocessor\MReid Projects\09st412\CARBON_01.fid\fid Frequency (MHz) 150.85 Nucleus 13C Number of Transients 2048 Original Points Count 32768 Points Count 262144 Pulse Sequence s2pul Receiver Gain 60.00 Solvent CHLOROFORM-d Spectrum Offset (Hz) 16433.1406 Spectrum Type STANDARD Sweep Width (Hz) 33472.80 Temperature (degree C) 25.000 6 O 7 10 8 9 5 4 3 2 14 13 12 11 O 16 CH2 17 O 1 CH3 18 H3C 15 184 176 168 160 152 144 136 128 120 112 104 96 88 80 72 64 56 48 40 32 24 16 8 0 Chemical Shift (ppm) 16.33(13) 17.94(18) 29.40(12) 23.84(15) 32.44(4) 36.94(10) 37.26(11) 37.56(14) 39.40(5) 61.05(2) 67.39(3) 75.04(6) 123.66(17) 136.57(9) 169.53(8) No. Atom Exp. Shift (ppm) 1 2 61.05 2 3 67.39 3 4 32.44 4 5 39.40 5 6 75.04 6 8 169.53 7 9 136.57 8 10 36.94 9 11 37.26 10 12 29.40 11 13 16.33 12 14 37.56 13 15 23.84 14 17 123.66 15 18 17.94 255 200 180 160 140 120 100 80 60 40 20 0 Chemical Shift (ppm) Chloroform-d 18.06 12.42 22.39 25.15 29.67 32.28 41.15 35.29 47.72 48.61 52.17 77.21 77.00 76.79 80.52 177.62 201.40 211.03 10 5 9 6 8 7 12 3 11 4 2 14 O 1 H O O CH3 15 O CH3 13 CDCl3 C-9 C-14 C-1 256 200 180 160 140 120 100 80 60 40 20 Chemical Shift (ppm) 18.09 12.46 22.41 25.20 29.70 32.30 35.32 41.17 47.76 48.64 80.54 52.19 201.46 48.5 Chemic...47.76 48.64 10 5 9 6 8 7 12 3 11 4 2 14 O 1 H O O CH3 15 O CH3 13 C-8 C-12 C-11 C-7 C-14 C-5 C-6 C-4 CH2 CH3 CH C-15 C C-13 -3 C-2 257 01/02/2006 14:48 06nk536 Dianne Irwin 0170771724 D:\nmr_data\06nk536C FW 306.4828 Formula C20H34O2 MS technique ESI + NMR solvent CDCl3 NMR experiment COSY;HMBC;HSQC/HMQC;NOESY;noe difference/noesy1D;1D Proton;1D Carbon Analytical number 06nk536 Assignment Service 6 5 7 10 8 9 4 3 2 1 H C3 20 CH3 19 18 OH 13 H C2 17 11 12 13 14 CH3 16 15 HO 20 150 145 140 135 130 125 120 115 110 105 100 95 90 85 80 75 70 65 60 55 50 45 40 35 30 25 20 15 10 Chemical Shift (ppm) 15.08(20) 16.48(16) 17.72(19) 18.80(2) 21.88(11) 24.28(6) 35.52(3) 38.17(7) 38.45(12) 38.67(1) 39.61(10) 48.58(5) 56.30(9) 106.57(17) 72.19(18) 59.56(15) 123.11(14) 148.39(8) 140.68(13) No. Atom Exp. Shift (ppm) 1 20 15.08 2 16 16.48 3 19 17.72 4 2 18.80 5 11 21.88 6 6 24.28 7 3 35.52 No. Atom Exp. Shift (ppm) 8 4 38.10 9 7 38.17 10 12 38.45 11 1 38.67 12 10 39.61 13 5 48.58 14 9 56.30 No. Atom Exp. Shift (ppm) 15 15 59.56 16 18 72.19 17 17 106.57 18 14 123.11 19 13 140.68 20 8 148.39 258
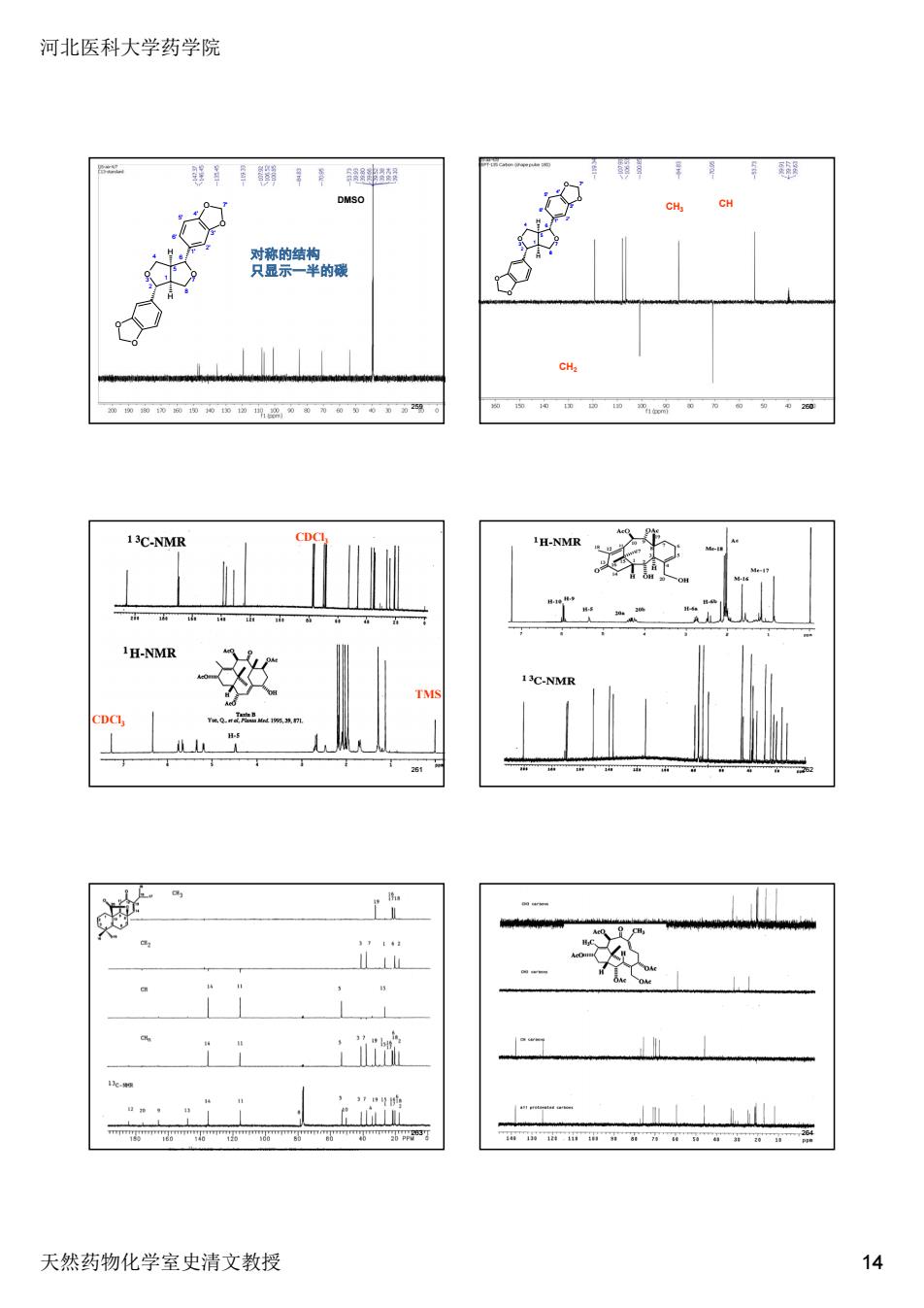
河北医科大学药学院 MS CH CH C-NM H-NMR 新可 天然药物化学室史清文教授 14
河北医科大学药学院 天然药物化学室史清文教授 14 2 O 3 1 4 5 8 O 7 6 1' 6' 2' 5' 3' 4' O O 7' O O H H DMSO 对称的结构 只显示一半的碳 259 2 O 3 1 4 5 8 O 7 6 1' 6' 2' 5' 3' 4' O O 7' O O H H CH2 CH3 CH 260 CDCl3 CDCl3 TMS 261 262 263 264

河北医科大学药学院 1211.DEPT C 核磁共振碳谱的解析 核磁共振碳谱的解析 解析步 2》计不 3)分于对称性的分新:著请假数目少子元素复高式 中暖原子的最目,说明分子有一定的对兼性: 、膜 刀对蒙由的结构逢行硬潘福认, The meo-like y--diol acc aide (SDA)adop Delayed COSY The a ide (AnA with C? re quivalent.In the SD are almost identical to each0时hcr(d2 5 ppm).As ane ring tend山sto pt a chair co )It sh etry clements in 1.2-di 天然药物化学室史清文教授 15
河北医科大学药学院 天然药物化学室史清文教授 15 265 266 核磁共振碳谱的解析 解析步骤: 1)区分出杂质峰、溶剂峰,不要遗漏季碳的谱线; ÿ)计算不饱和度 Ā)分子对称性的分析:若谱线数目少于元素组成式 中碳原子的数目,说明分子有一定的对称性; 4)碳原子级数的确定(活泼氢数目的确定); 5)碳原子d值的分区:饱和区、不饱和区、杂原子区、羰基区 6)推出结构单元,组合可能的结构式; 7)对推出的结构进行碳谱指认。 267 核磁共振碳谱的解析 Fig. 1. Conformations, chemical shifts, and NOES for (a) syn-1,3-diol acetonide (SDA) and (b) anti-1,3-diol acetonide (ADA). 268 • The meso-like syn-1,3-diol acetonide (SDA), adopts a chair 1,3-dioxolane conformation with axial and equatorial methyl groups. The anti-1,3-diol acetonide (ADA) with C2- like symmetry, prefers the twist-boat conformation to minimize 1,3-diaxial interactions; and the methyl groups are almost equivalent. In the SDA, the axial and equatorial methyl groups resonate atwd 30 ppm and wd 20 ppm, respectively. In the ADA case, the methyl group chemical shifts are almost identical to each other (wd 25 ppm). As the size difference of substituents at C4 and C6 increase, the 1,3-dioxane ring tends to adopt a chair conformation (Fig. 1). It should be noted that the symmetry elements in 1,2-diol acetonides are also useful for assignment purposes. 269 Delayed COSY Delayed COSY allows linking of signals which are very weakly coupled (down to 0.1 Hz) Journal of Ethnopharm. 1991, 32, 103-110.270