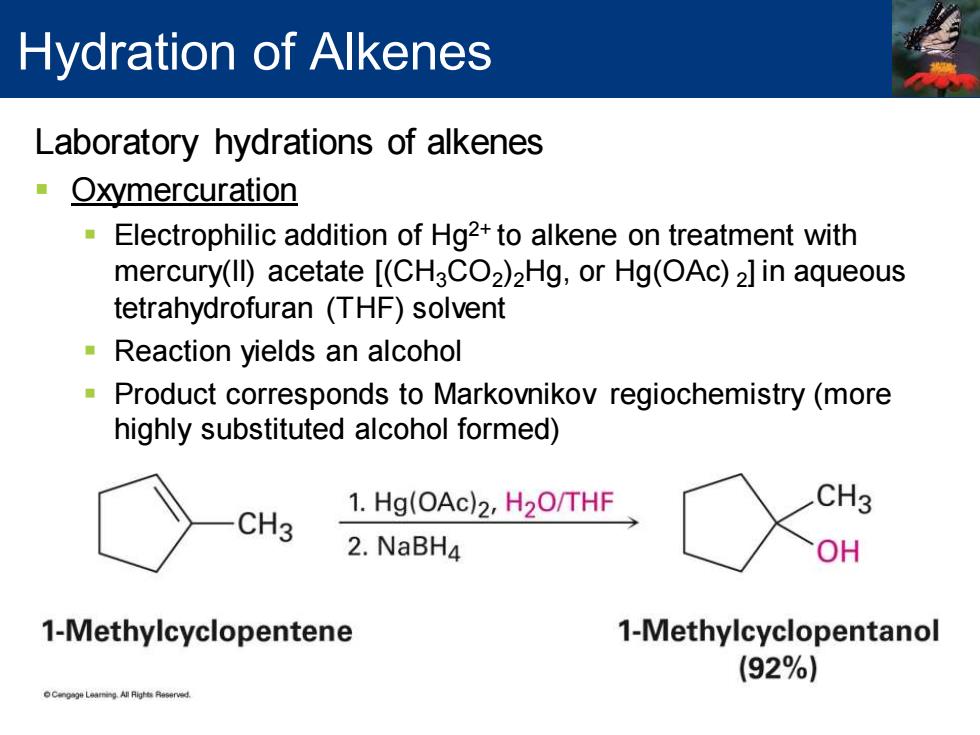
Hydration of Alkenes Laboratory hydrations of alkenes Oxymercuration Electrophilic addition of Hg2+to alkene on treatment with mercury(Il)acetate [(CH3CO2)2Hg,or Hg(OAc)2]in aqueous tetrahydrofuran (THF)solvent Reaction yields an alcohol Product corresponds to Markovnikov regiochemistry(more highly substituted alcohol formed) CH3 1.Hg(OAc)2,H2O/THF CH3 2.NaBH4 OH 1-Methylcyclopentene 1-Methylcyclopentanol (92%)
Laboratory hydrations of alkenes ▪ Oxymercuration ▪ Electrophilic addition of Hg2+ to alkene on treatment with mercury(II) acetate [(CH3CO2 )2Hg, or Hg(OAc) 2 ] in aqueous tetrahydrofuran (THF) solvent ▪ Reaction yields an alcohol ▪ Product corresponds to Markovnikov regiochemistry (more highly substituted alcohol formed) Hydration of Alkenes
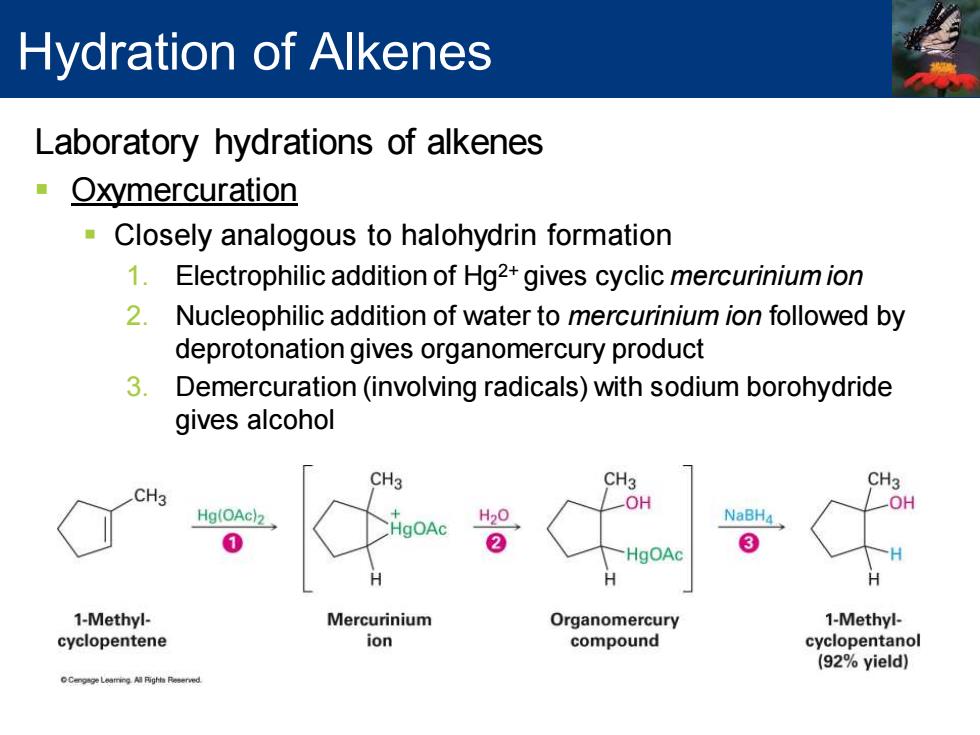
Hydration of Alkenes Laboratory hydrations of alkenes Oxymercuration Closely analogous to halohydrin formation 1.Electrophilic addition of Hg2+gives cyclic mercurinium ion 2.Nucleophilic addition of water to mercurinium ion followed by deprotonation gives organomercury product 3. Demercuration(involving radicals)with sodium borohydride gives alcohol CH3 CH3 CH3 CH3 OH OH Hg(OAc)2 NaBH4 0 2 -HgOAc 3 H 1-Methyl- Mercurinium Organomercury 1-Methyl- cyclopentene ion compound cyclopentanol (92%yield)
Laboratory hydrations of alkenes ▪ Oxymercuration ▪ Closely analogous to halohydrin formation 1. Electrophilic addition of Hg2+ gives cyclic mercurinium ion 2. Nucleophilic addition of water to mercurinium ion followed by deprotonation gives organomercury product 3. Demercuration (involving radicals) with sodium borohydride gives alcohol Hydration of Alkenes

Hydration of Alkenes Hydroboration/oxidation Addition of a B-H bond of borane,BH3,to an alkene Occurs in single step No carbocation intermediate Reaction yields an alcohol Syn stereochemistry Both C-H and C-B bonds form at the same time and from the same face of the double-bond Product has non-Markovnikov regiochemistry CH3 CH3 BH3 H202,0HF THF solvent -BH2 OH 1-Methyl- Organoborane trans-2-Methyl- cyclopentene intermediate cyclopentanol (85%yield) DCmpoLa Nhp Finat
▪ Hydroboration/oxidation ▪ Addition of a B-H bond of borane, BH3 , to an alkene ▪ Occurs in single step ▪ No carbocation intermediate ▪ Reaction yields an alcohol ▪ Syn stereochemistry ▪ Both C-H and C-B bonds form at the same time and from the same face of the double-bond ▪ Product has non-Markovnikov regiochemistry Hydration of Alkenes
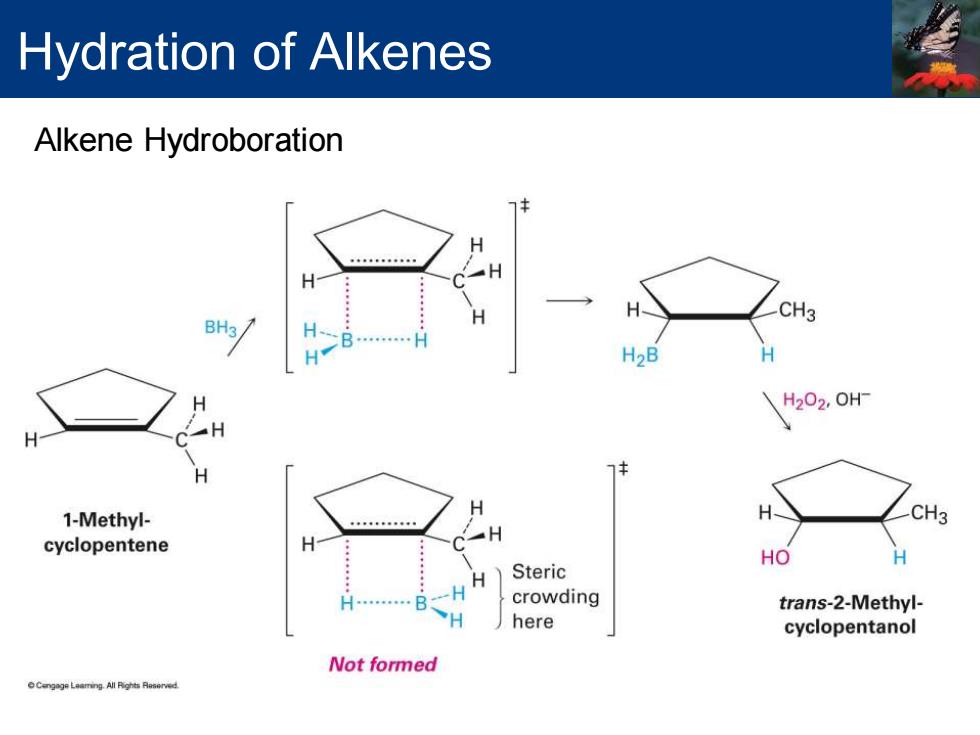
Hydration of Alkenes Alkene Hydroboration H CH3 BH3/ H2B H H202,0H 1-Methyl- H CH3 cyclopentene HO Steric H crowding trans-2-Methyl- here cyclopentanol Not formed Cengge Laaming All Rights Raserved
Alkene Hydroboration Hydration of Alkenes

Worked Example 8.1 Predicting the Products of a Hydration Reaction What products would you obtain from reaction of 2- methylpent-2-ene with: (a)BH3,followed by H2O2,OH (b)Hg(OAc)2,followed by NaBH4
What products would you obtain from reaction of 2- methylpent-2-ene with: (a) BH3 , followed by H2O2 ,OH- (b) Hg(OAc)2 , followed by NaBH4 Worked Example 8.1 Predicting the Products of a Hydration Reaction