
Solved Problem 3-1 Give the structures of 4-isopropyloctane and 5-t-butyldecane. Solution:4-lsopropyloctane has a chain of eight carbons,with an isopropyl group on the fourth carbon.5-t-Butyldecane has a chain of ten carbons, with a t-butyl group on the fifth. CH,-CH-CH, CH,-CH,-CH,-CH-CH,-CH,-CH,-CH, 4-isopropyloctane CH, CH,-C-CH, CH,-CH,-CH,-CH,一CH-CH,-CH,-CH,一CH,-CH 5-t-butyldecane Chapter 3 16
Chapter 3 16 Solution: 4-Isopropyloctane has a chain of eight carbons, with an isopropyl group on the fourth carbon. 5-t-Butyldecane has a chain of ten carbons, with a t-butyl group on the fifth. Solved Problem 3-1 Give the structures of 4-isopropyloctane and 5-t-butyldecane
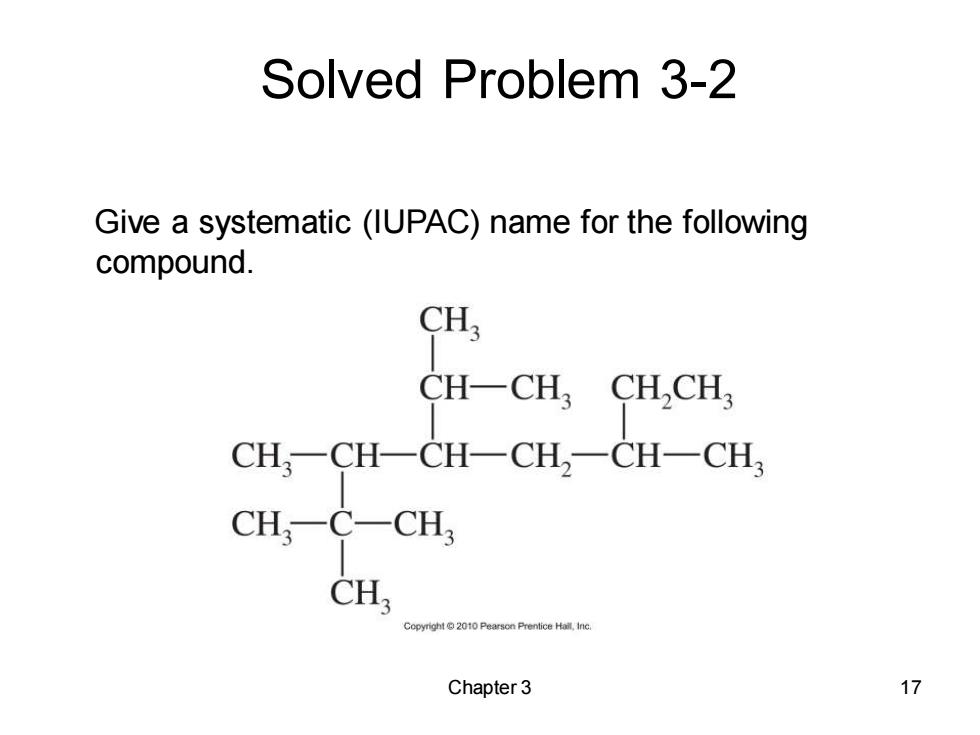
Solved Problem 3-2 Give a systematic (IUPAC)name for the following compound. CH; CH-CH CHCH3 CH,一CH一CH一CH2一CH-CH CH,-C-CH, CH Chapter 3 17
Chapter 3 17 Solved Problem 3-2 Give a systematic (IUPAC) name for the following compound

Solved Problem 3-2:Solution The longest carbon chain contains eight carbon atoms,so this compound is named as an octane.Numbering from left to right gives the first branch on C2;numbering from right to left gives the first branch on C3,so we number from left to right. CH; CH一CH, CHCH, CH, CH-4CH-CH,-CH-CH3 CH; CH CH C0200 4-isopropyl-2,2,3,6-tetramethyloctane Chapter 3 18
Chapter 3 18 The longest carbon chain contains eight carbon atoms, so this compound is named as an octane. Numbering from left to right gives the first branch on C2; numbering from right to left gives the first branch on C3, so we number from left to right. Solved Problem 3-2: Solution 4-isopropyl-2,2,3,6-tetramethyloctane

Boiling Points of Alkanes As the number of carbons in an alkane increases,the boiling point will increase due to the larger surface area and the increased van der Waals attractions. 400 300 CH3一(CH2n一CH 200 n-alkanes 100 CH3 0 CH一(CH2)nCH3 100 isoalkanes CH3 -200 10 15 20 number of carbon atoms Copyright 2010 Pearson Prentice Hall,Inc. Chapter 3 19
Chapter 3 19 Boiling Points of Alkanes As the number of carbons in an alkane increases, the boiling point will increase due to the larger surface area and the increased van der Waals attractions
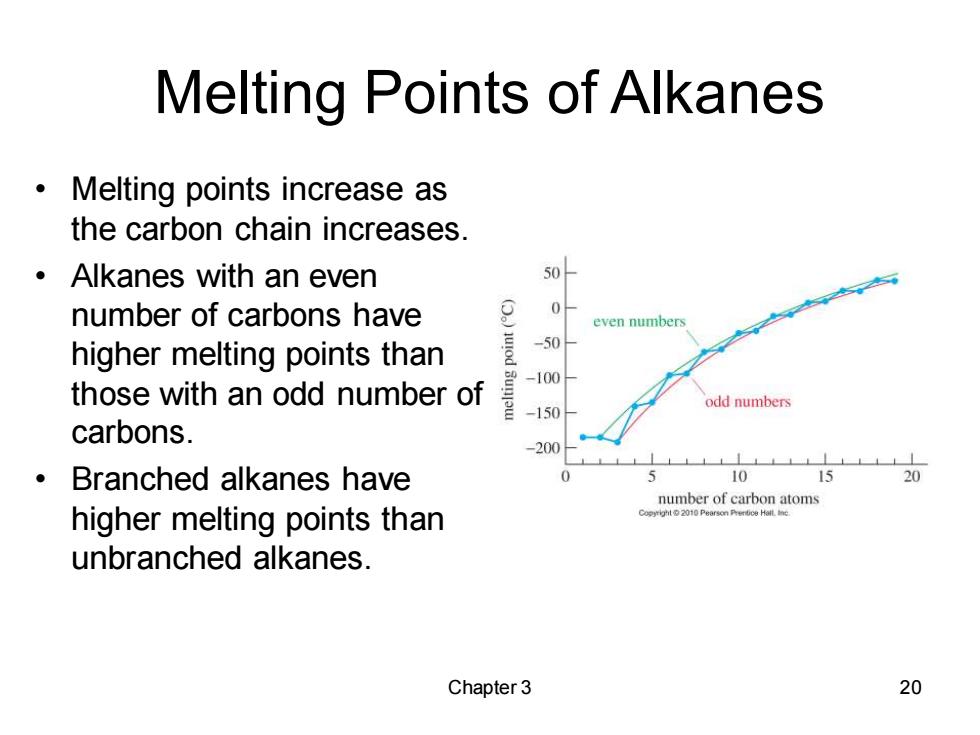
Melting Points of Alkanes Melting points increase as the carbon chain increases. ·Alkanes with an even 50 number of carbons have 0 even numbers higher melting points than 50 -100 those with an odd number of odd numbers -150 carbons. -200 Branched alkanes have 5 10 15 20 number of carbon atoms higher melting points than o01 Pearsono Hall unbranched alkanes. Chapter 3 20
Chapter 3 20 Melting Points of Alkanes • Melting points increase as the carbon chain increases. • Alkanes with an even number of carbons have higher melting points than those with an odd number of carbons. • Branched alkanes have higher melting points than unbranched alkanes