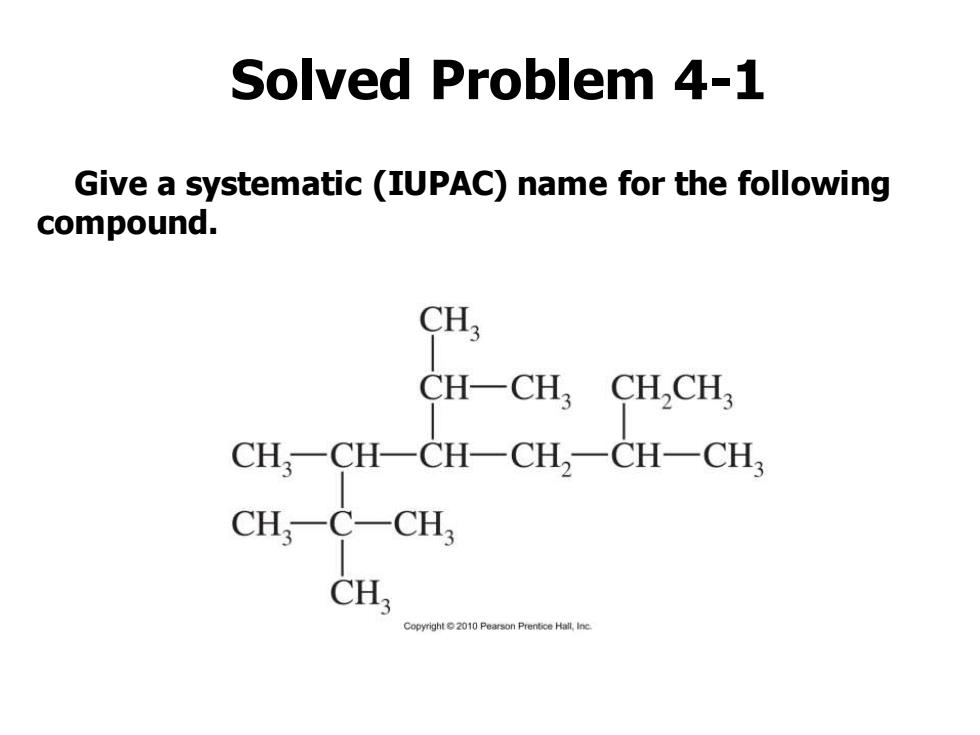
Solved Problem 4-1 Give a systematic (IUPAC)name for the following compound. CH; CH-CH CHCH CH一CH一CH-CH2一CH-CH CHCCH, CH Copyright2010 Pearson Prentice Hall,Ine
Solved Problem 4-1 Give a systematic (IUPAC) name for the following compound
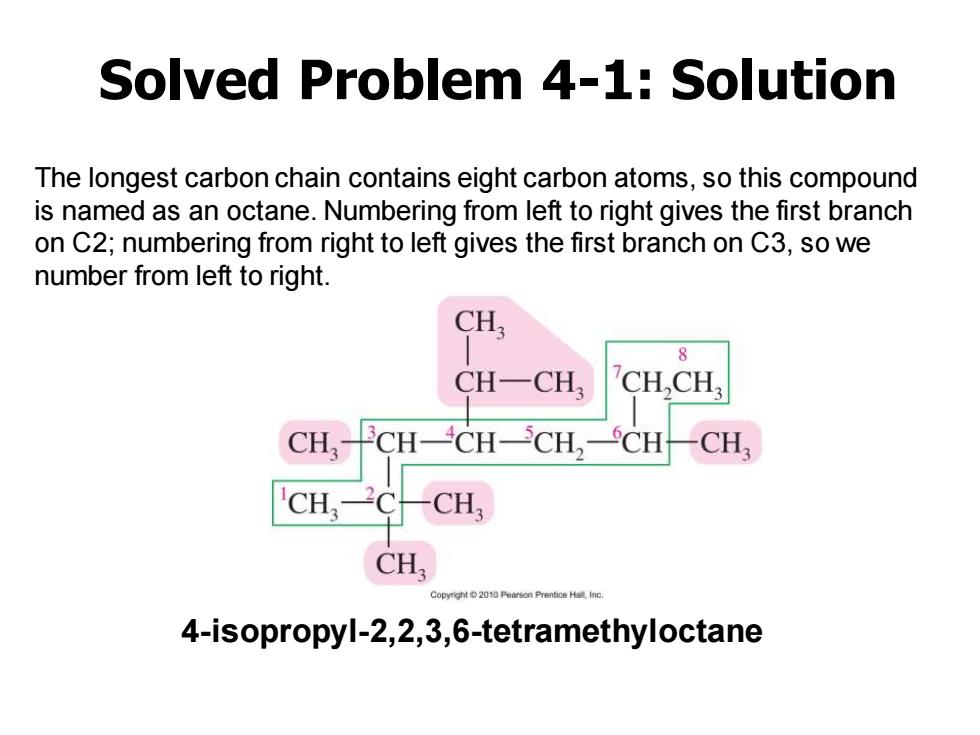
Solved Problem 4-1:Solution The longest carbon chain contains eight carbon atoms,so this compound is named as an octane.Numbering from left to right gives the first branch on C2;numbering from right to left gives the first branch on C3,so we number from left to right. CH 8 CH-CH, CH,CH, CH,- CH-CH-CH,-CH-CH3 CH CH CH Copyrgnt 2010 4-isopropyl-2,2,3,6-tetramethyloctane
The longest carbon chain contains eight carbon atoms, so this compound is named as an octane. Numbering from left to right gives the first branch on C2; numbering from right to left gives the first branch on C3, so we number from left to right. Solved Problem 4-1: Solution 4-isopropyl-2,2,3,6-tetramethyloctane

SEC 1 Physical Properties Solubility:hydrophobic Density:less than 1 g/mL Boiling points increase with increasing carbons (little less for branched chains). Melting points increase with increasing carbons(less for odd-number of carbons)
SEC 1 Physical Properties ◼Solubility: hydrophobic ◼Density: less than 1 g/mL ◼Boiling points increase with increasing carbons (little less for branched chains). Melting points increase with increasing carbons (less for odd-number of carbons)
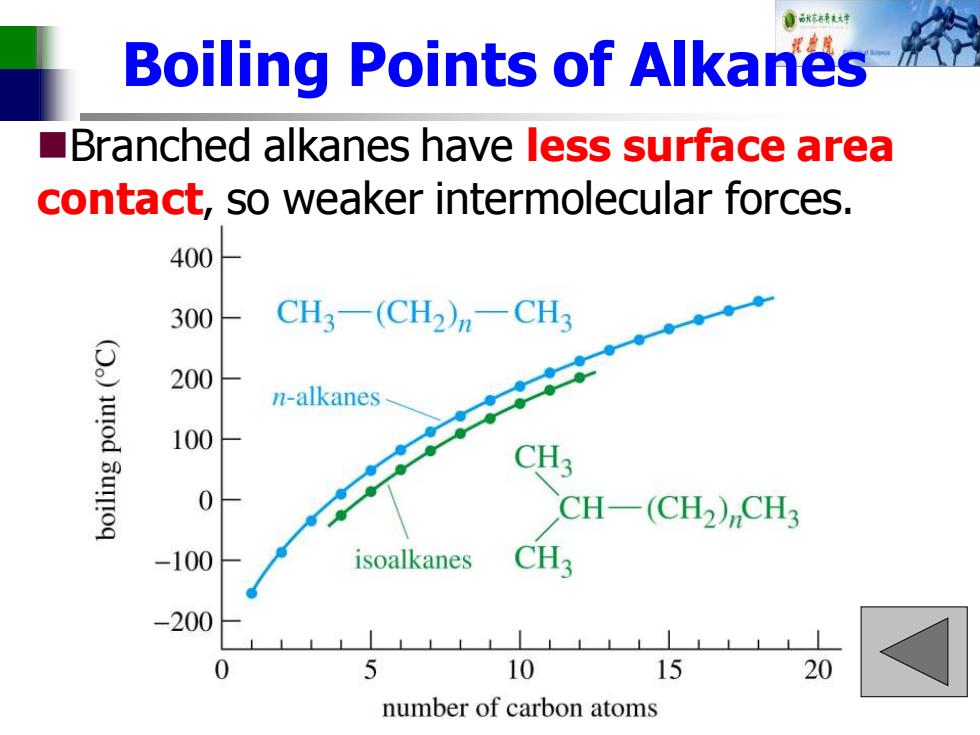
自秋不转大对 Boiling Points of Alkanes ■ Branched alkanes have less surface area contact,so weaker intermolecular forces. 400 300 CH3-(CH2)n-CH3 ()uod 3um!oq 200 n-alkanes 100 0 CH-(CH2)CH3 100 isoalkanes CH3 -200 5 10 15 20 number of carbon atoms
Boiling Points of Alkanes ◼Branched alkanes have less surface area contact, so weaker intermolecular forces
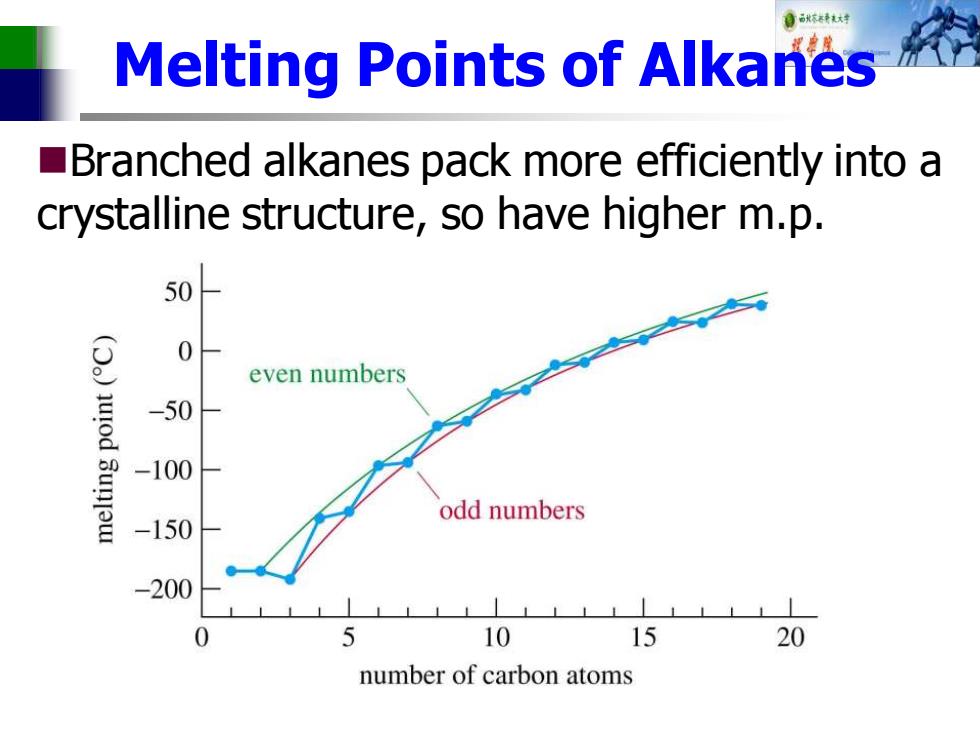
自秋不特大对 Melting Points of Alkanes Branched alkanes pack more efficiently into a crystalline structure,so have higher m.p. 50 0 even numbers -50 -100 -150 odd numbers -200 0 5 10 15 20 number of carbon atoms
Melting Points of Alkanes ◼Branched alkanes pack more efficiently into a crystalline structure, so have higher m.p