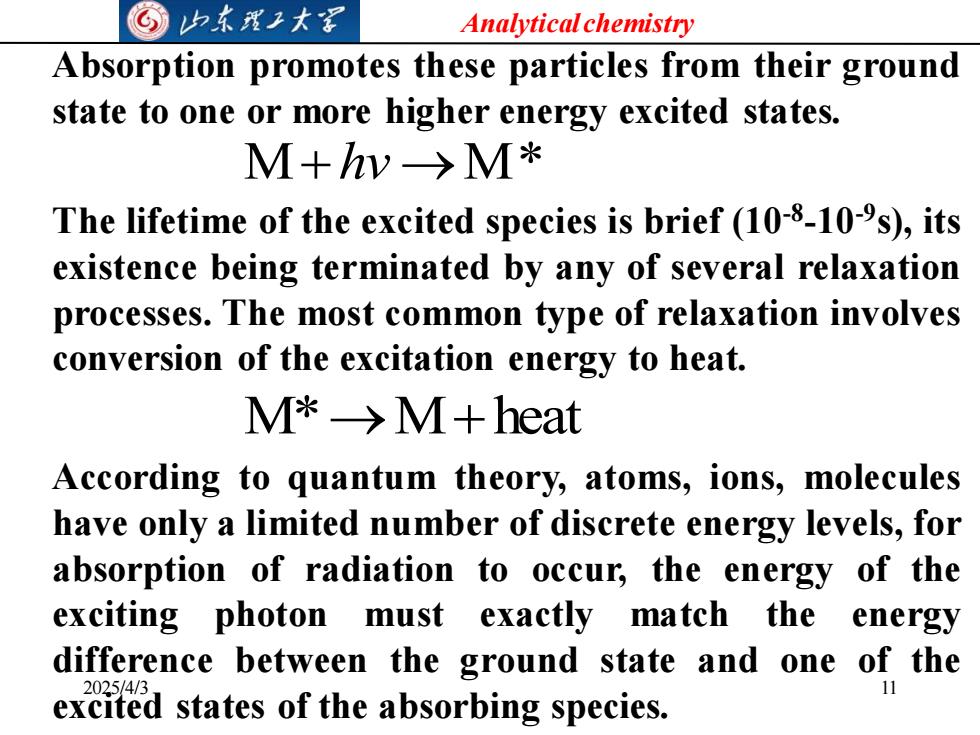
G山本理子大军 Analytical chemistry Absorption promotes these particles from their ground state to one or more higher energy excited states. M+hw→M* The lifetime of the excited species is brief(10-8-10-s),its existence being terminated by any of several relaxation processes.The most common type of relaxation involves conversion of the excitation energy to heat. M*->M+heat According to quantum theory,atoms,ions,molecules have only a limited number of discrete energy levels,for absorption of radiation to occur,the energy of the exciting photon must exactly match the energy difference between the ground state and one of the 2025/4/ excited states of the absorbing species
Analytical chemistry 2025/4/3 11 Absorption promotes these particles from their ground state to one or more higher energy excited states. M+hv →M* The lifetime of the excited species is brief (10-8 -10-9 s), its existence being terminated by any of several relaxation processes. The most common type of relaxation involves conversion of the excitation energy to heat. M*→M+heat According to quantum theory, atoms, ions, molecules have only a limited number of discrete energy levels, for absorption of radiation to occur, the energy of the exciting photon must exactly match the energy difference between the ground state and one of the excited states of the absorbing species

©山东置王大 Analytical chemistry Since these energy differences are unique for each species,a study of the frequencies of absorbed radiation provides a means of characterizing the constituents of a sample of matter.For this purpose,a plot of absorbance as a function of wavelenth or frequency is experimentally derived. Cr202- MnO 2025/4/3 300350400525 700(nm) 12
Analytical chemistry 2025/4/3 12 Since these energy differences are unique for each species, a study of the frequencies of absorbed radiation provides a means of characterizing the constituents of a sample of matter. For this purpose, a plot of absorbance as a function of wavelenth or frequency is experimentally derived. Cr2O7 2- MnO4 -
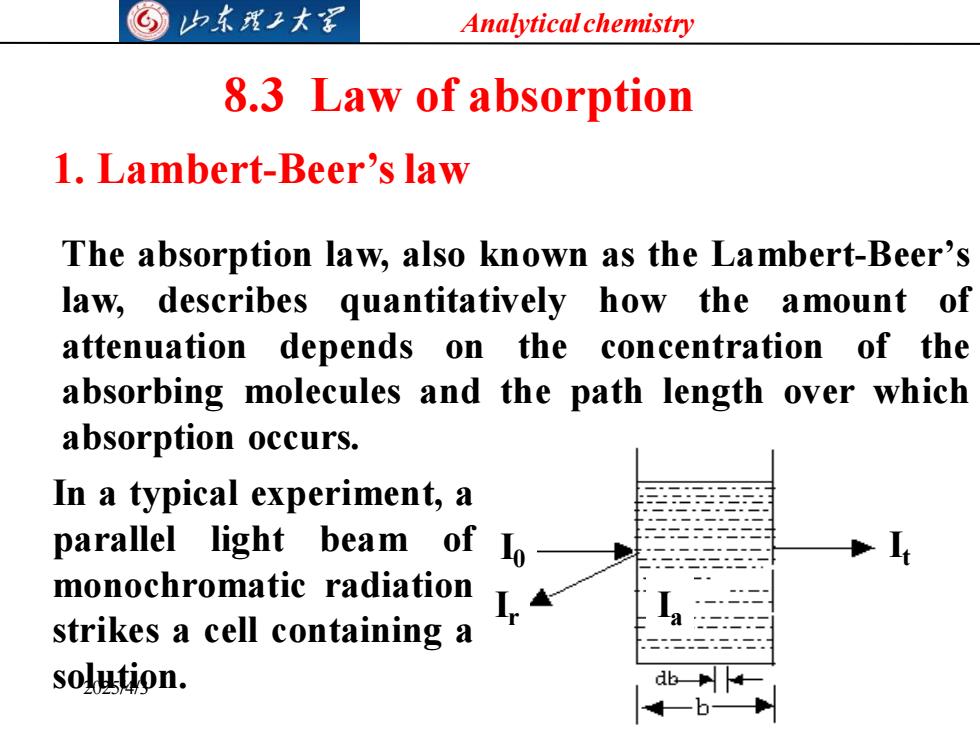
归东理2大军 Analytical chemistry 8.3 Law of absorption 1.Lambert-Beer's law The absorption law,also known as the Lambert-Beer's law,describes quantitatively how the amount of attenuation depends on the concentration of the absorbing molecules and the path length over which absorption occurs. In a typical experiment,a parallel light beam of Io monochromatic radiation strikes a cell containing a solution
Analytical chemistry 2025/4/3 13 8.3 Law of absorption 1. Lambert-Beer’slaw The absorption law, also known as the Lambert-Beer’s law, describes quantitatively how the amount of attenuation depends on the concentration of the absorbing molecules and the path length over which absorption occurs. I0 Ir It Ia In a typical experiment, a parallel light beam of monochromatic radiation strikes a cell containing a solution

G归东理工大图 Analytical chemistry After passing through the cell,the radiation power of the beam decreases from Io to Ie due to reflection losses at the cell windows,absorption in the sample and by scattering at dispersed particles.Only the absorption losses are caused by the dissolved sample. The operation is repeated using an identical cell containing only solvent to compensate for reflection and scattering losses,then the transmittance T is calculated using the following equation. 1 T 10 solvent 2025/4/3 14
Analytical chemistry 2025/4/3 14 After passing through the cell, the radiation power of the beam decreases from I0 to It , due to reflection losses at the cell windows, absorption in the sample and by scattering at dispersed particles. Only the absorption losses are caused by the dissolved sample. The operation is repeated using an identical cell containing only solvent to compensate for reflection and scattering losses, then the transmittance T is calculated using the following equation. solvent solution 0 I I I I T t =
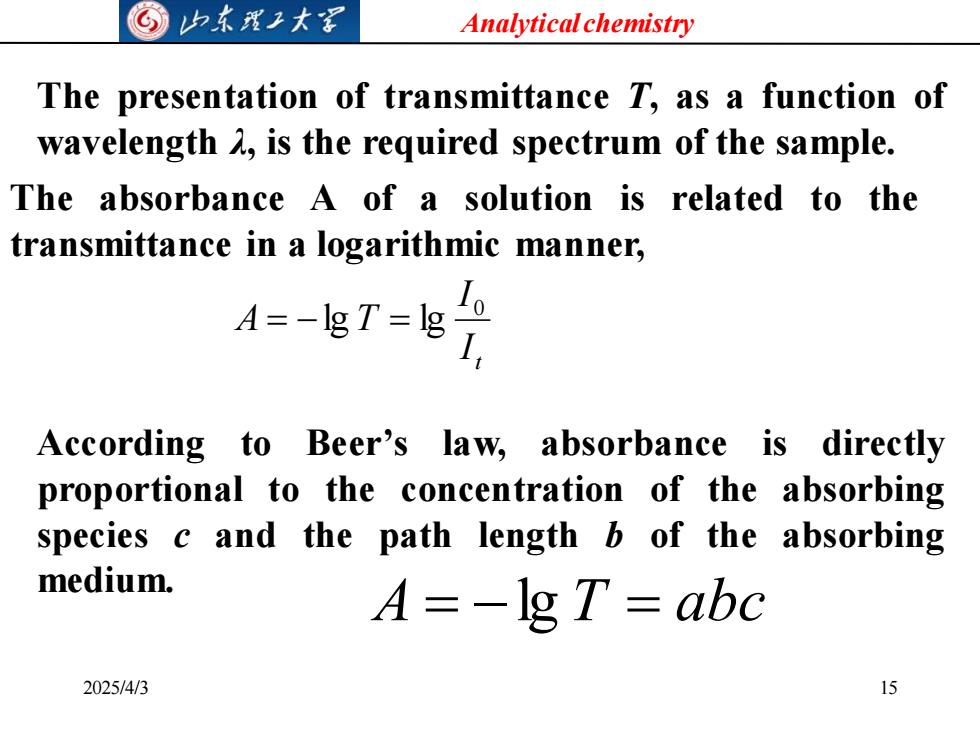
©山东理王大写 Analytical chemistry The presentation of transmittance T,as a function of wavelength A,is the required spectrum of the sample. The absorbance a of a solution is related to the transmittance in a logarithmic manner, 4=-T-E According to Beer's law,absorbance is directly proportional to the concentration of the absorbing species c and the path length b of the absorbing medium. A=-1g T=abc 2025/4/3 15
Analytical chemistry 2025/4/3 15 The presentation of transmittance T, as a function of wavelength λ, is the required spectrum of the sample. The absorbance A of a solution is related to the transmittance in a logarithmic manner, t I I A T 0 = −lg = lg According to Beer’s law, absorbance is directly proportional to the concentration of the absorbing species c and the path length b of the absorbing medium. A = −lg T = abc