
G归东理工大 Analytical chemistry Here,a is a proportionality constant called the absorptivity,if c has the units of g/L and b has the unit of cm,absorptiviy has the unit of L/g.cm. When the concentration is expressed in moles per liter and b in centimeters,the proportionality constant is called the molar absorptivity and is given the special symbol,&and its unit is L/mol.cm. A-8bc 2025/4/3 16
Analytical chemistry 2025/4/3 16 Here, a is a proportionality constant called the absorptivity, if c has the units of g/L and b has the unit of cm, absorptiviy has the unit of L/g·cm. When the concentration is expressed in moles per liter and b in centimeters, the proportionality constant is called the molar absorptivity and is given the special symbol, ε, and its unit is L/mol·cm. A=bc
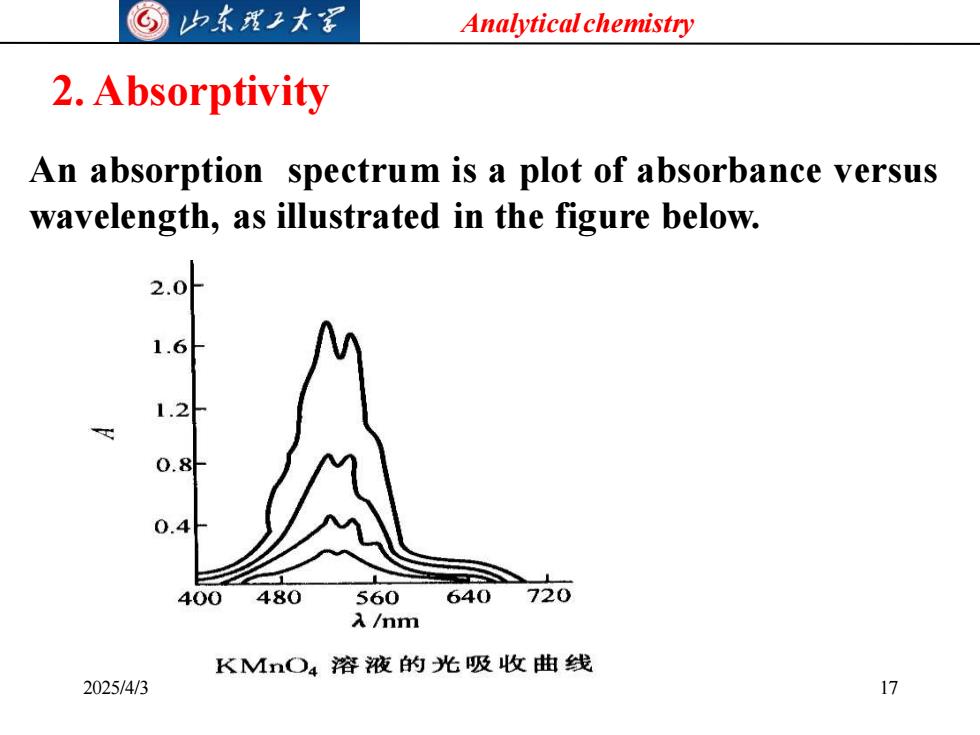
G山本理子大军 Analytical chemistry 2.Absorptivity An absorption spectrum is a plot of absorbance versus wavelength,as illustrated in the figure below. 2.0 6 0.8 400 480 560640 720 入/mm KMnO4溶液的光吸收曲线 2025/4/3 17
Analytical chemistry 2025/4/3 17 2. Absorptivity An absorption spectrum is a plot of absorbance versus wavelength, as illustrated in the figure below

©归东卫大军 Analytical chemistry A plot molar absorptivity cas a function of wavelength is independent of concentration.This type of spectral plot is characteristic for a given molecule and is sometimes used to aid to indetifying or confirming the identity of a particular species. The abosrptivity a or molar absorptivity a is characteristic of a particular combination of solute and solvent for a given wavelength. It must be noted carefully that abosrptivity is a substance,whereas absorbance is a property of a particular sample and will therefore vary with the concentration and the length of the light path through then container. 2025/4/3 18
Analytical chemistry 2025/4/3 18 A plot molar absorptivity εas a function of wavelength is independent of concentration. This type of spectral plot is characteristic for a given molecule and is sometimes used to aid to indetifying or confirming the identity of a particular species. The abosrptivity a or molar absorptivity ε is characteristic of a particular combination of solute and solvent for a given wavelength. It must be noted carefully that abosrptivity is a substance, whereas absorbance is a property of a particular sample and will therefore vary with the concentration and the length of the light path through then container