
G归东理工大图 Analytical chemistry In some respects its properties are those of wave while in others it appears to consist of a series of discrete particles of energy,called photons. Both wavelength and wave number are dependent on the refractive index of the medium through which the radiation is passing,whereas the frequency is independent of this property. V= -=VC 2025/4/3
Analytical chemistry 2025/4/3 6 In some respects its properties are those of wave ,while in others it appears to consist of a series of discrete particles of energy, called photons. Both wavelength and wave number are dependent on the refractive index of the medium through which the radiation is passing, whereas the frequency is independent of this property. c c v = =
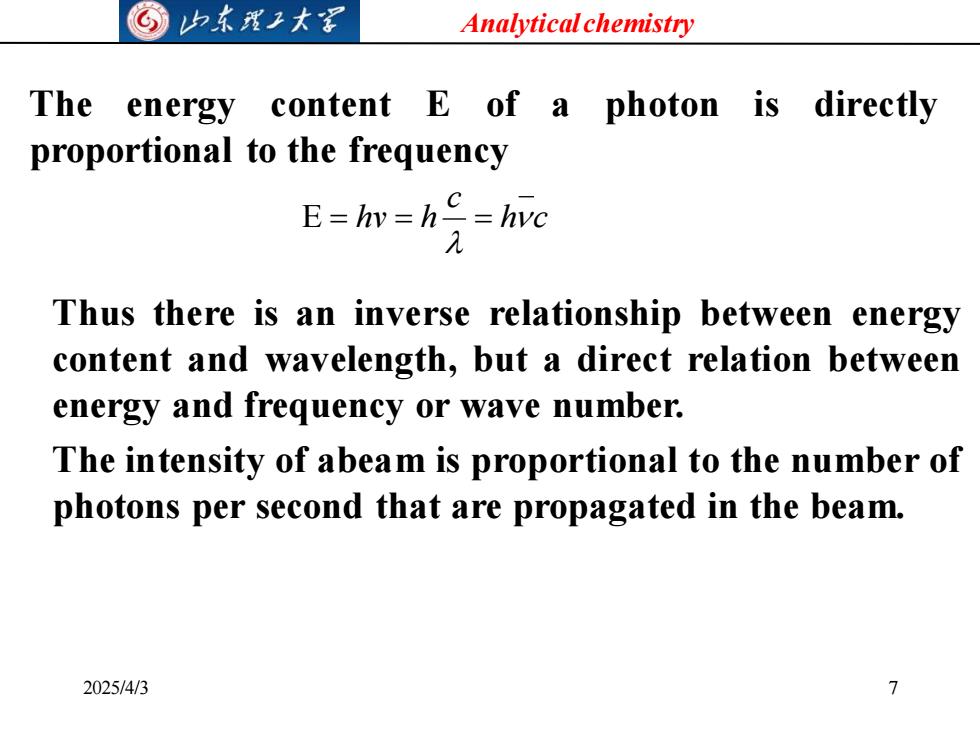
©归东子大写 Analytical chemistry The energy content E of a photon is directly proportional to the frequency E=hy=h=hvc Thus there is an inverse relationship between energy content and wavelength,but a direct relation between energy and frequency or wave number. The intensity of abeam is proportional to the number of photons per second that are propagated in the beam. 2025/4/3 7
Analytical chemistry 2025/4/3 7 The energy content E of a photon is directly proportional to the frequency h c c hv h E = = = Thus there is an inverse relationship between energy content and wavelength, but a direct relation between energy and frequency or wave number. The intensity of abeam is proportional to the number of photons per second that are propagated in the beam
©山东理卫大 Analytical chemistry 2.Selective absorption of radiation and absorbance spectrum The term spectroscopy was originally used to describe a branch of science based upon the resolution of visible radiation into its component wavelengths. If a beam of white light passes through a glass container filled with liquid,the emergent radiation is always less powerful than that entering.The diminution of power is generally of different extent for different wavelengths. 2025/4/3
Analytical chemistry 2025/4/3 8 2. Selective absorption of radiation and absorbance spectrum The term spectroscopy was originally used to describe a branch of science based upon the resolution of visible radiation into its component wavelengths. If a beam of white light passes through a glass container filled with liquid, the emergent radiation is always less powerful than that entering. The diminution of power is generally of different extent for different wavelengths

©归东龙工大写 Analytical chemistry The loss is due in part to reflections at the surfaces and to scattering by any suspended particles present,but in the absence of such particles,it is primarily accounted for by the absorption of radiant energy by the liquid. If the energy absorbed is greater for some visible wavelengths than for others,the emergent beam will appear colored.The apparent color of the solution is always the complement of the color absorbed. A solution absorbing in the blue region will appear yellow,one that absorbs green will appear purple. 2025/4/3 9
Analytical chemistry 2025/4/3 9 The loss is due in part to reflections at the surfaces and to scattering by any suspended particles present, but in the absence of such particles, it is primarily accounted for by the absorption of radiant energy by the liquid. If the energy absorbed is greater for some visible wavelengths than for others, the emergent beam will appear colored. The apparent color of the solution is always the complement of the color absorbed. A solution absorbing in the blue region will appear yellow, one that absorbs green will appear purple

Analytical chemistry The general term for chemical analysis through the measurement of absorption of radiation is absorptiometry.The term colorimetry applies only in relation to the visible region.Spectrophotometry is a division of absorptiometry that refers specifically to the use of a spectrophotometer. When radiation passes through a layer of material, certain frequencies may be selectively removed by absorption,a process in which electromagnetic energy is transferred to the atoms,ions,or molecules constituting the sample. 2025/4/3 10
Analytical chemistry 2025/4/3 10 The general term for chemical analysis through the measurement of absorption of radiation is absorptiometry. The term colorimetry applies only in relation to the visible region. Spectrophotometry is a division of absorptiometry that refers specifically to the use of a spectrophotometer. When radiation passes through a layer of material, certain frequencies may be selectively removed by absorption, a process in which electromagnetic energy is transferred to the atoms, ions, or molecules constituting the sample