The rate of reaction depends on (among other factors)... Concentration of some or all of the molecules present 。Temperature The presence of a catalyst Rate equation:f(Ca,t,T)=0 At constant T:f (CB,t)=0 rate-concentration---Differential NO NO. concentration-time---Integrated PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
The rate of reaction depends on (among other factors) ... · Concentration of some or all of the molecules present · Temperature · The presence of a catalyst Rate equation: f(CB,t,T)=0 At constant T: f(CB,t)=0 rate-concentration--- Differential concentration-time---Integrated PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn _
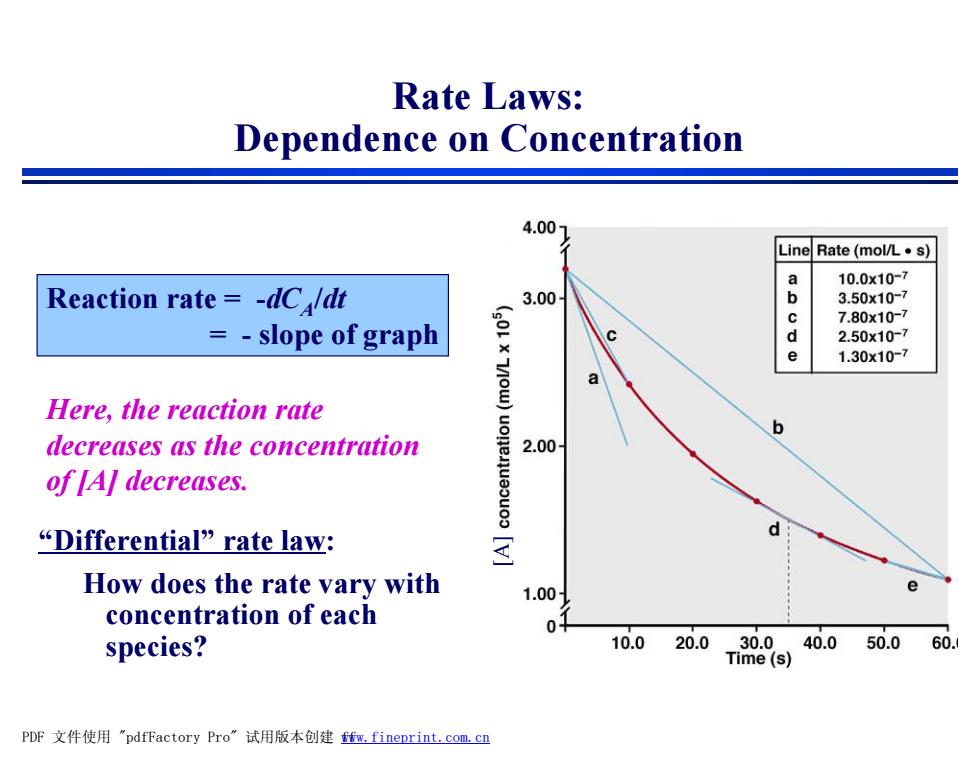
Rate Laws: Dependence on Concentration 4.00 Line Rate(mo/L·s) a 10.0x10-7 Reaction rate -dC/dt 3.00 b 3.50x10-7 c 7.80x10-7 =-slope of graph d 2.50x10-7 e 1.30x10-7 Here,the reaction rate b decreases as the concentration 2.00 of [A]decreases. “Differential'”rate law: d How does the rate vary with 1.00 concentration of each 0 species? 10.0 20.030.040.050.060. Time(s) PDF文件使用"pdfFactory Pro”试用版本创建ffm,fineprint,com,cn
Rate Laws: Dependence on Concentration Reaction rate = -dCA /dt = - slope of graph Here, the reaction rate decreases as the concentration of [A] decreases. [A “D ] ifferential” rate law: How does the rate vary with concentration of each species? PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f
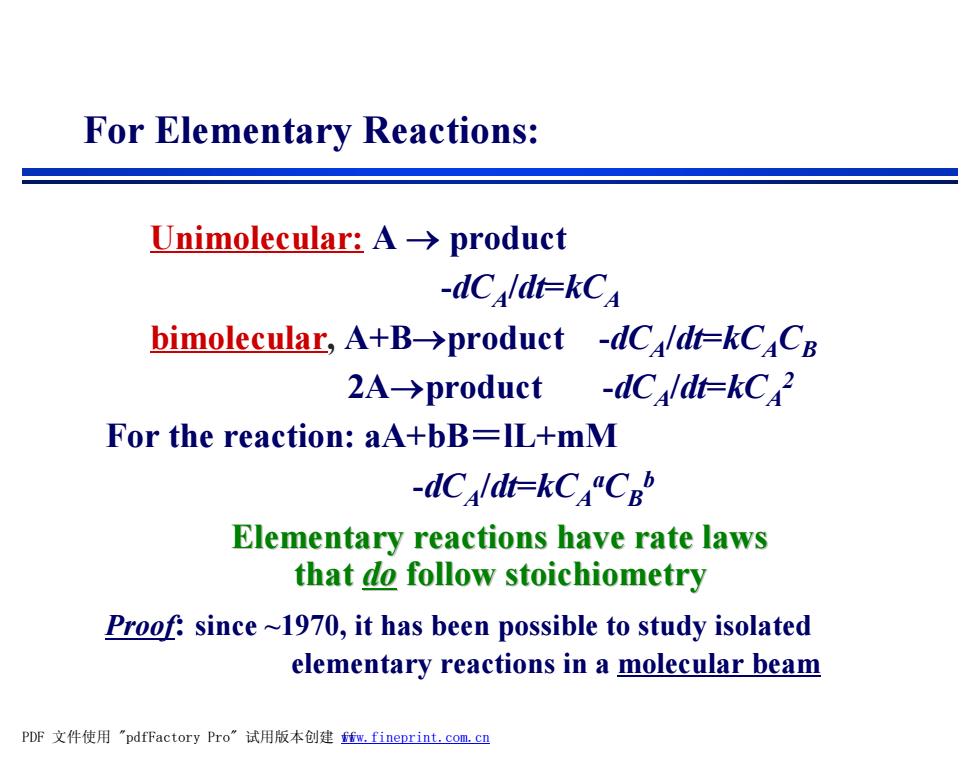
For Elementary Reactions: Unimolecular:A->product -dCldi=kCA bimolecular,A+B->product -dC/dt=kCaCB 2A→product -dCaldt=kC2 For the reaction:aA+bB=IL+mM -dCaldt=kCCb Elementary reactions have rate laws that do follow stoichiometry Proof:since~1970,it has been possible to study isolated elementary reactions in a molecular beam PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
Unimolecular: A ® product -dCA /dt=kCA bimolecular, A+B®product -dCA /dt=kCACB 2A®product -dCA /dt=kCA 2 For the reaction: aA+bB=lL+mM -dCA /dt=kCA aCB b Elementary reactions have rate laws that do follow stoichiometry Proof: since ~1970, it has been possible to study isolated elementary reactions in a molecular beam For Elementary Reactions: PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f
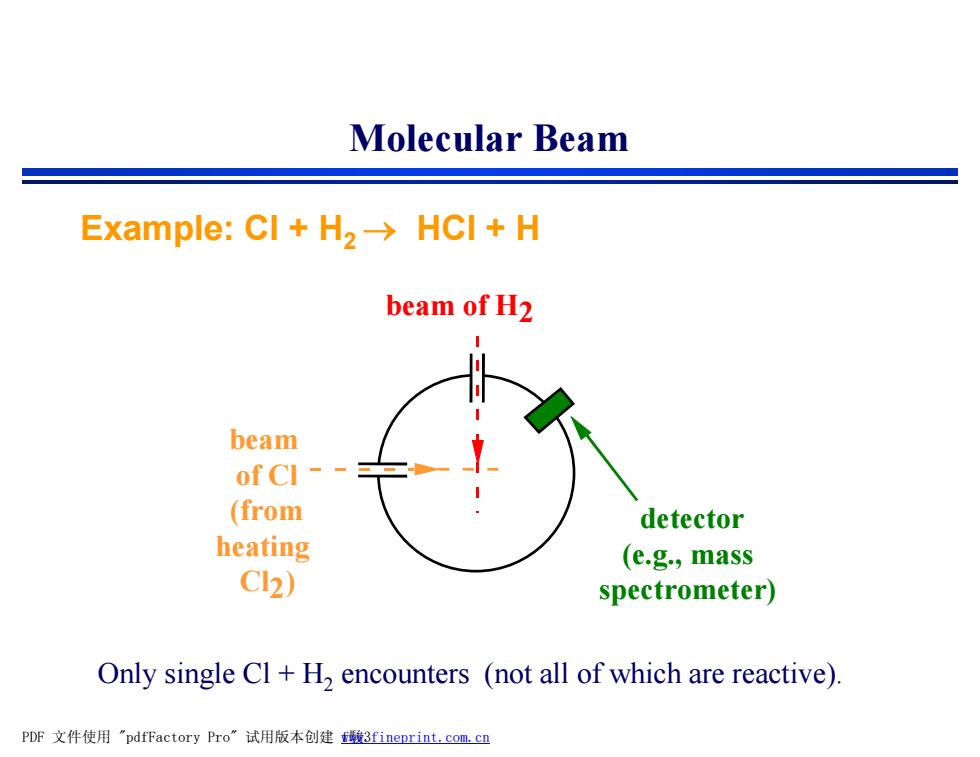
Molecular Beam Example:CI+H2->HCI H beam of H2 beam of CI - (from detector heating (e.g.,mass Cl2) spectrometer) Only single Cl H,encounters (not all of which are reactive). PDF文件使用"pdfFactory Pro”试用版本创建曦3 fineprint.com,cn
Only single Cl + H2 encounters (not all of which are reactive). beam of H2 beam of Cl (from heating Cl2) detector (e.g., mass spectrometer) Example: Cl + H2 ® HCl + H Molecular Beam PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn 駿3
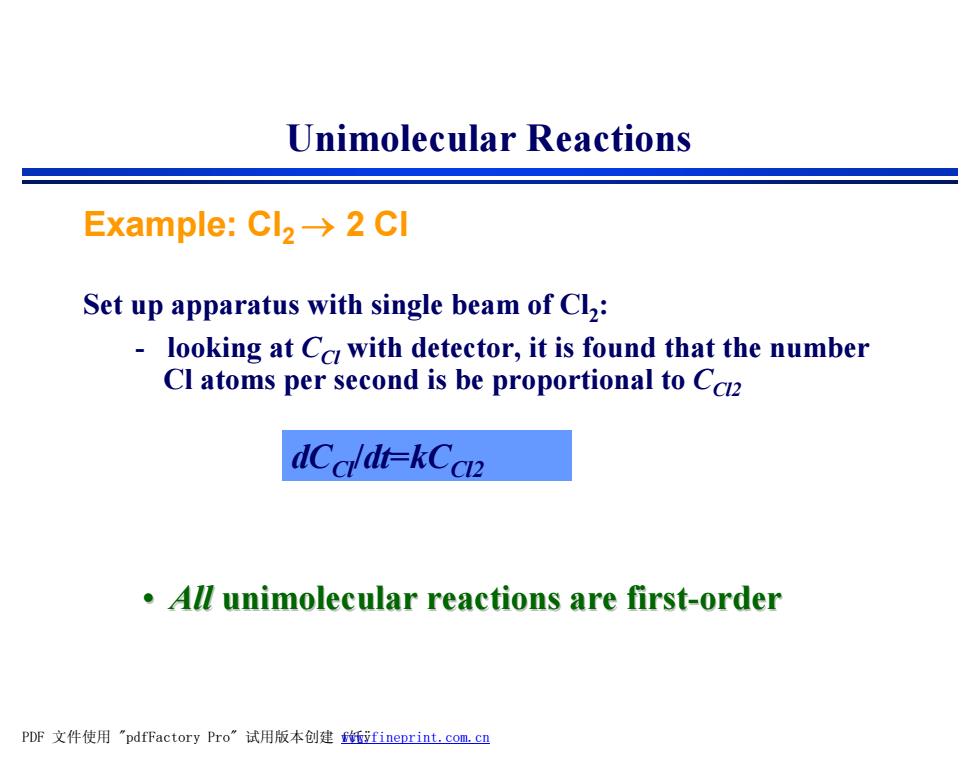
Unimolecular Reactions Example:Cl2-→2Cl Set up apparatus with single beam of Cl2: looking at Cc with detector,it is found that the number Cl atoms per second is be proportional to Cc2 dCald+-kCcn2 All unimolecular reactions are first-order PDF文件使用"pdfFactory Pro”试用版本创建伍ifineprint..com,cn
Example: Cl2 ® 2 Cl Set up apparatus with single beam of Cl2 : - looking at CCl with detector, it is found that the number Cl atoms per second is be proportional to CCl2 • All unimolecular reactions are first-order Unimolecular Reactions dCCl/dt=kCCl2 PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn 饦ÿ