Chemical equilibrium PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com,c四
Chemical equilibrium PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

Introduction Chemical reaction moved towards a dynamic equilibrium in which both reactants and products are present but have no further tendency to undergo net change.In some cases,the concentration of products in the equilibrium mixture is so much greater than the concentration of unchanged reactants that for all practical purposes the reaction is 'complete'. However,in many important cases the equilibrium mixture has significant concentration of both reactants and products.In this chapter we see how to use thermodynamics to predict the equilibrium composition under any reaction conditions. PDF文件使用"pdfFactory Pro”试用版本创建w.fineprint.com.cn
Introduction Chemical reaction moved towards a dynamic equilibrium in which both reactants and products are present but have no further tendency to undergo net change. In some cases, the concentration of products in the equilibrium mixture is so much greater than the concentration of unchanged reactants that for all practical purposes the reaction is ‘complete’. However, in many important cases the equilibrium mixture has significant concentration of both reactants and products. In this chapter we see how to use thermodynamics to predict the equilibrium composition under any reaction conditions. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

Spontaneous chemical reactions We have seen that the direction of spontaneous change at constant temperature and pressure is towards lower values of the Gibbs energy G.The idea is entirely general, and in this chapter we apply it to the discussion of reaction. PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com,cn
Spontaneous chemical reactions We have seen that the direction of spontaneous change at constant temperature and pressure is towards lower values of the Gibbs energy ,G. The idea is entirely general, and in this chapter we apply it to the discussion of reaction. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

.The reaction Gibbs energy Consider the reaction:0=EveB At constant T and P,suppose the reaction advances by d,the corresponding change in Gibbs energy: dGT.p=MAdna+ugdng+ucdnc+.....=>uedng =LA VAdg+uB ved+uc vcd+......=>vB uBds dGT,p=∑%ed5 .(OGla)1p=ΣB4B=△,Gm The reaction Gibbs energy,A,Gm is defined as the slope of the graph of the Gibbs energy plotted against the extent of reaction. PDF文件使用"pdfFactory Pro”试用版本创建耀m,fineprint.com,cn
Consider the reaction: 0=ånBB At constant T and P,suppose the reaction advances by dx,the corresponding change in Gibbs energy: dGT,P=mAdnA+mBdnB+mCdnC+......=åmBdnB =mAnAdx+mBnBdx+mCnCdx+......=ånB mBdx dGT,P=ånB mBdx \(¶G/¶x)T,P=ånB mB=DrGm •The reaction Gibbs energy The reaction Gibbs energy, DrGm, is defined as the slope of the graph of the Gibbs energy plotted against the extent of reaction. PDF 文件使用 "pdfFactory Pro" 试用版本创建 耀www.fineprint.com.cn
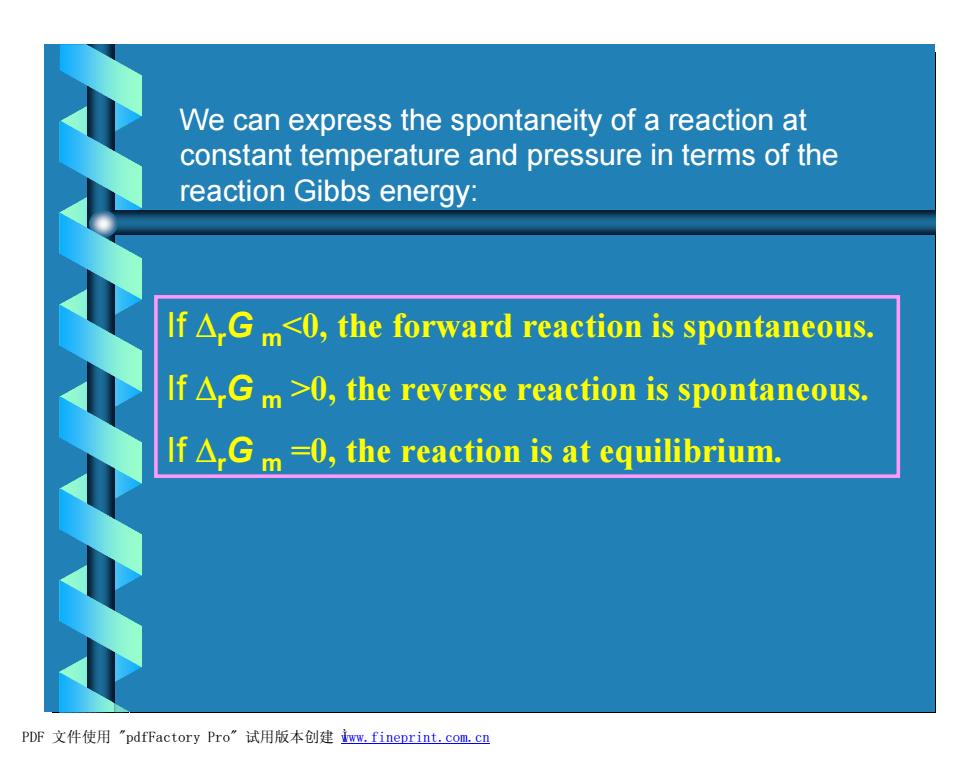
We can express the spontaneity of a reaction at constant temperature and pressure in terms of the reaction Gibbs energy: If A.Gm<0,the forward reaction is spontaneous. If A.Gm>0,the reverse reaction is spontaneous. If△,Gm-0,the reaction is at equilibrium. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
We can express the spontaneity of a reaction at constant temperature and pressure in terms of the reaction Gibbs energy: If DrG m<0, the forward reaction is spontaneous. If DrG m >0, the reverse reaction is spontaneous. If DrG m =0, the reaction is at equilibrium. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn