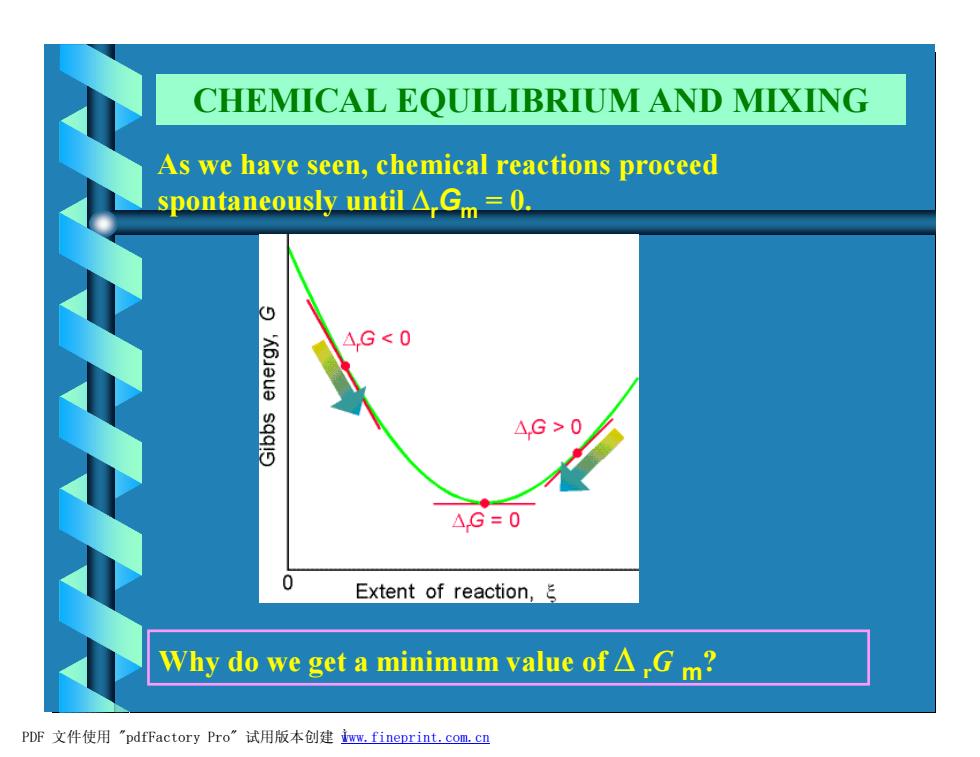
CHEMICAL EQUILIBRIUM AND MIXING As we have seen,chemical reactions proceed spontaneously until A,Gm =0. △,G<0 △,G>0 △.G=0 0 Extent of reaction, Why do we get a minimum value of△,Gm? PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint..com,cn
As we have seen, chemical reactions proceed spontaneously until DrGm = 0. Why do we get a minimum value of D rG m? CHEMICAL EQUILIBRIUM AND MIXING PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
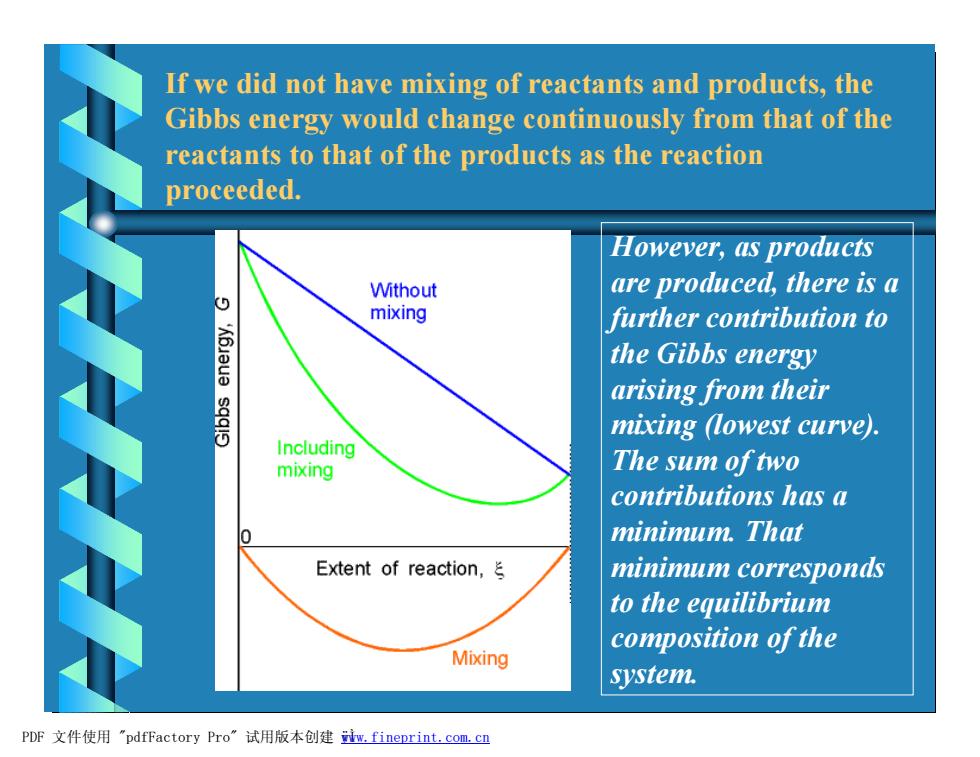
If we did not have mixing of reactants and products,the Gibbs energy would change continuously from that of the reactants to that of the products as the reaction proceeded. However,as products Without are produced,there is a mixing further contribution to the Gibbs energy arising from their sqqis mixing (lowest curve) Including mixing The sum oftwo contributions has a minimum.That Extent of reaction, minimum corresponds to the equilibrium composition of the Mixing system. PDF文件使用"pdfFactory Pro”试用版本创建iw,fineprint.com,cn
If we did not have mixing of reactants and products, the Gibbs energy would change continuously from that of the reactants to that of the products as the reaction proceeded. However, as products are produced, there is a further contribution to the Gibbs energy arising from their mixing (lowest curve). The sum of two contributions has a minimum. That minimum corresponds to the equilibrium composition of the system. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì
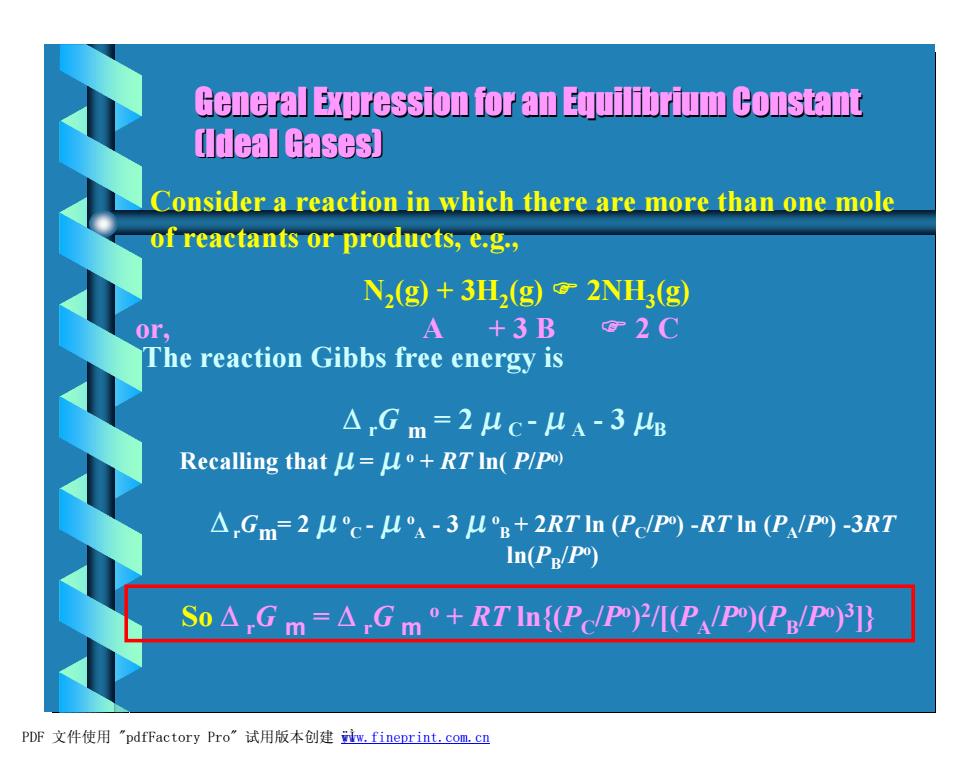
General Expression for an Equilibrium Constant Ildeal Gases】 Consider a reaction in which there are more than one mole of reactants or products,e.g., N2(g)+3H(g)2NH3(g) 0r, A+3B2C The reaction Gibbs free energy is △Gm=2uc-4A-3E Recalling that u=uo+RT In(P/PO) △,Gm=2uc-uA-3u°g+2RTin(Pcpm)-RT In(PPO))-3RT In(Pg/po) So△Gm=△Gm'+RTIn{(PP)2M(PAP)(PB/P PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com.c四
General Expression for an Equilibrium Constant (Ideal Gases) Consider a reaction in which there are more than one mole of reactants or products, e.g., N2 (g) + 3H2 (g) F 2NH3 (g) or, A + 3 B F 2 C The reaction Gibbs free energy is D rG m = 2 m C - m A - 3 mB Recalling that m = m o + RT ln( P/Po) DrGm= 2 m o C - m o A - 3 m o B + 2RT ln (PC /Po ) -RT ln (PA /Po ) -3RT ln(PB /Po ) So D rG m = D rG m o + RT ln{(PC /Po ) 2 /[(PA /Po )(PB /Po ) 3 ]} PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì
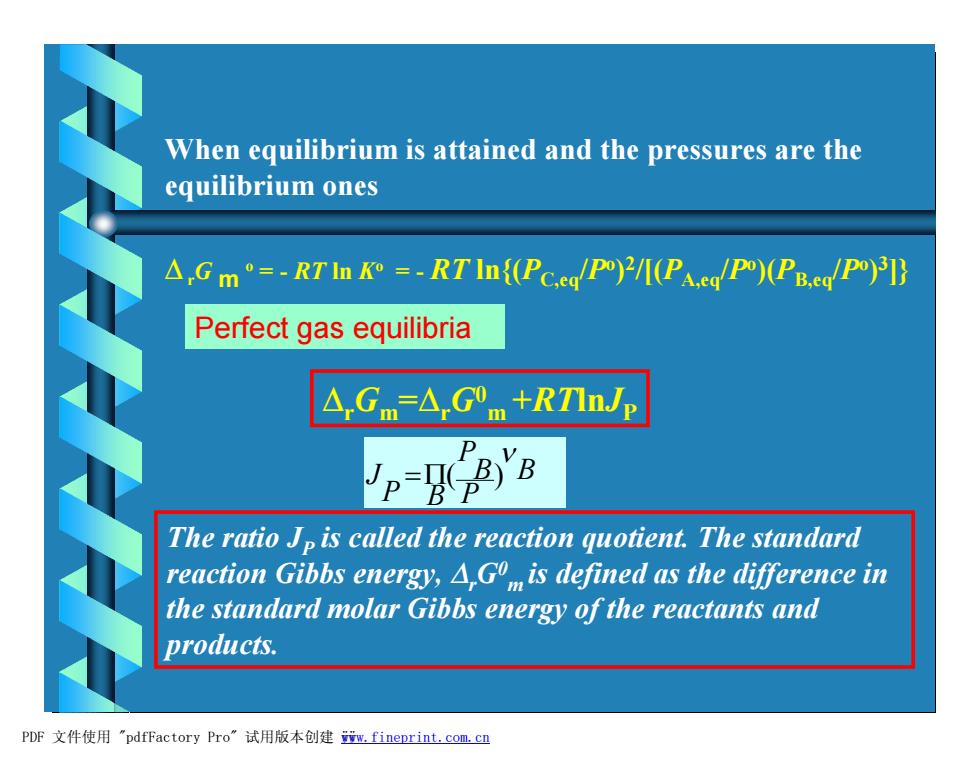
When equilibrium is attained and the pressures are the equilibrium ones △,Gm°=-RTn9=-RTln{(Pc.eg/PI(Pa.eg/P)PB.ed/P)]} Perfect gas equilibria △,Gm=△,Gm+RTlnJp Vo-o8 D The ratio Jp is called the reaction quotient.The standard reaction Gibbs energy,A,Go is defined as the difference in the standard molar Gibbs energy of the reactants and products. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com.c四
When equilibrium is attained and the pressures are the equilibrium ones DrG m o = - RT ln Ko = - RT ln{(PC,eq/Po ) 2 /[(PA,eq/Po )(PB,eq/Po ) 3 ]} DrGm =DrG0 m +RTlnJP Perfect gas equilibria ( ) B P B P P B J n =P The ratio JP is called the reaction quotient. The standard reaction Gibbs energy, DrG0 m is defined as the difference in the standard molar Gibbs energy of the reactants and products. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn ÿ
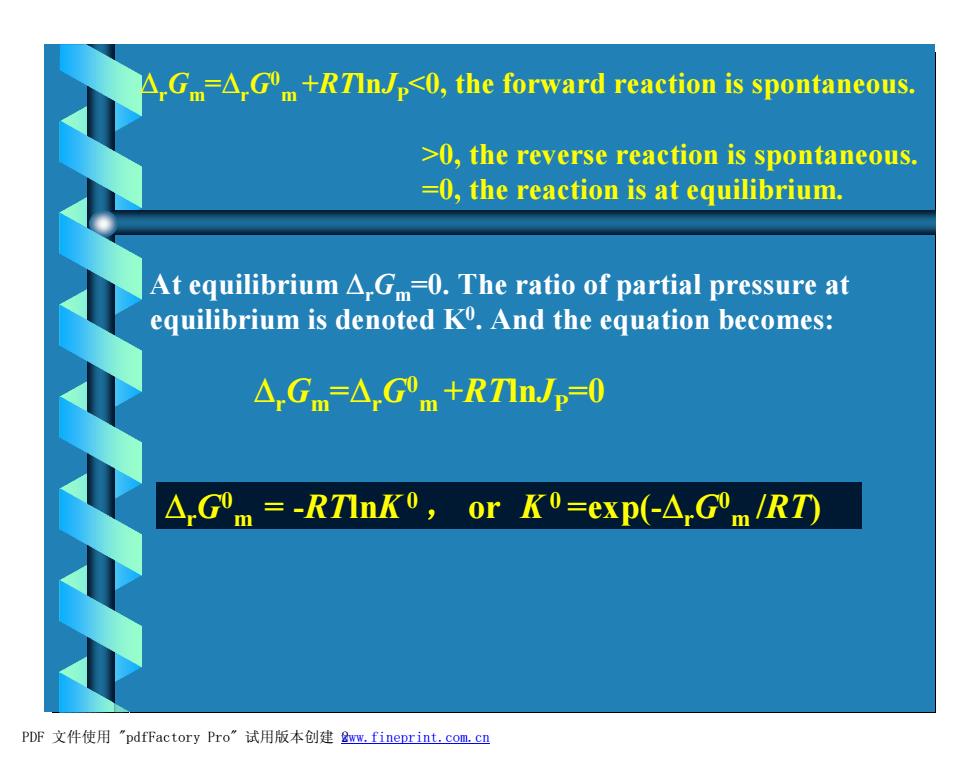
△,Gm△,G"m+RTInJp0,the forward reaction is spontaneous.. >0,the reverse reaction is spontaneous. =0,the reaction is at equilibrium. At equilibrium A.G=0.The ratio of partial pressure at equilibrium is denoted K.And the equation becomes: △Gm=△Gm+RTInJp=0 A.Gm=-RTInK0,or K0=exp(-AGOm/RT) PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
DrGm =DrG0 m +RTlnJP<0, the forward reaction is spontaneous. >0, the reverse reaction is spontaneous. =0, the reaction is at equilibrium. At equilibrium DrGm=0. The ratio of partial pressure at equilibrium is denoted K0 . And the equation becomes: DrGm =DrG0 m +RTlnJP=0 f f f f DrG0 m = -RTlnK0 , or K 0 =exp(-DrG0 m /RT) PDF 文件使用 "pdfFactory Pro" 试用版本创建 2www.fineprint.com.cn