Contents The perfect gas 1.1 The states of gases 1.2 The gas laws Real gases 1.3 Molecular interactions 1.4 The Van der Waals equation 1.5 The principle of corresponding states PDF文件使用"pdfFactory Pro”试用版本创建mm,fineprint,.com,c四
2 Contents The perfect gas 1.1 The states of gases 1.2 The gas laws Real gases 1.3 Molecular interactions 1.4 The Van der Waals equation 1.5 The principle of corresponding states PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

The properties of gases This chapter establishes the properties of gases that will be used throughout the text.It begins with an account of an idealized version of a gas,perfect gas,and shows how its equation of state may be assembled experimentally.We then see how the properties of real gases differ from those of a perfect gas and construct an equation of state that describes their properties. PDF文件使用"pdfFactory Pro”试用版本创建脑m,fineprint.com,c里
3 The properties of gases This chapter establishes the properties of gases that will be used throughout the text. It begins with an account of an idealized version of a gas, perfect gas, and shows how its equation of state may be assembled experimentally. We then see how the properties of real gases differ from those of a perfect gas , and construct an equation of state that describes their properties. PDF 文件使用 "pdfFactory Pro" 试用版本创建 嘀www.fineprint.com.cn
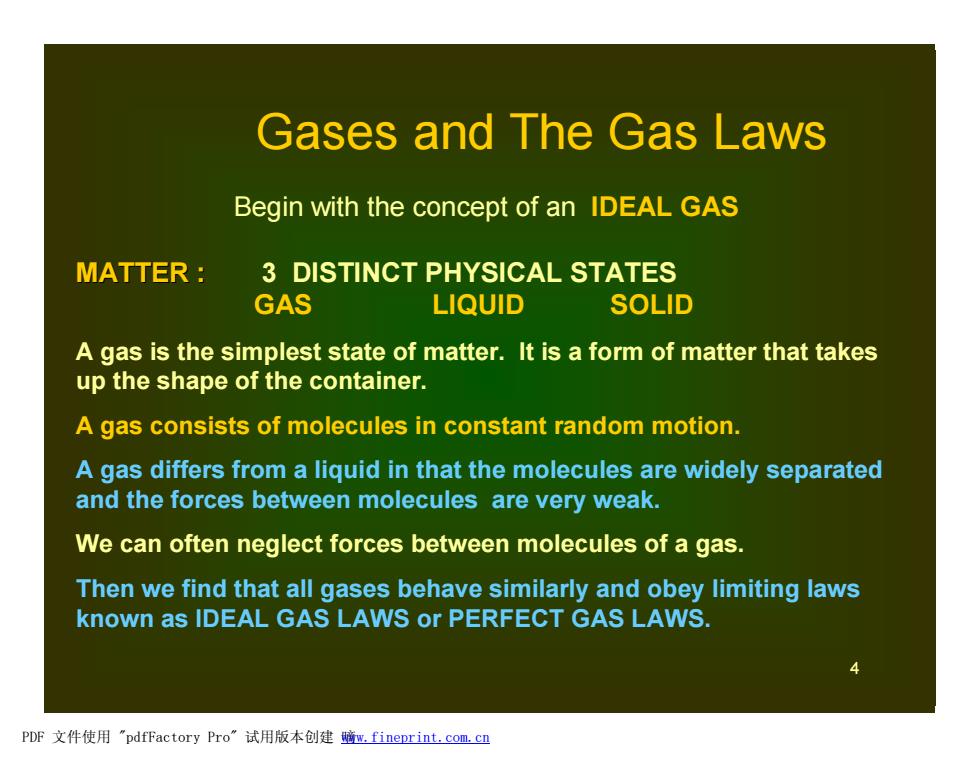
Gases and The Gas Laws Begin with the concept of an IDEAL GAS MATTER 3 DISTINCT PHYSICAL STATES GAS LIQUID SOLID A gas is the simplest state of matter.It is a form of matter that takes up the shape of the container. A gas consists of molecules in constant random motion. A gas differs from a liquid in that the molecules are widely separated and the forces between molecules are very weak. We can often neglect forces between molecules of a gas. Then we find that all gases behave similarly and obey limiting laws known as IDEAL GAS LAWS or PERFECT GAS LAWS. PDF文件使用"pdfFactory Pro”试用版本创建鳍m,fineprint.com,cn
4 Begin with the concept of an IDEAL GAS MATTER : 3 DISTINCT PHYSICAL STATES GAS LIQUID SOLID A gas is the simplest state of matter. It is a form of matter that takes up the shape of the container. A gas consists of molecules in constant random motion. A gas differs from a liquid in that the molecules are widely separated and the forces between molecules are very weak. We can often neglect forces between molecules of a gas. Then we find that all gases behave similarly and obey limiting laws known as IDEAL GAS LAWS or PERFECT GAS LAWS. Gases and The Gas Laws PDF 文件使用 "pdfFactory Pro" 试用版本创建 嘀www.fineprint.com.cn
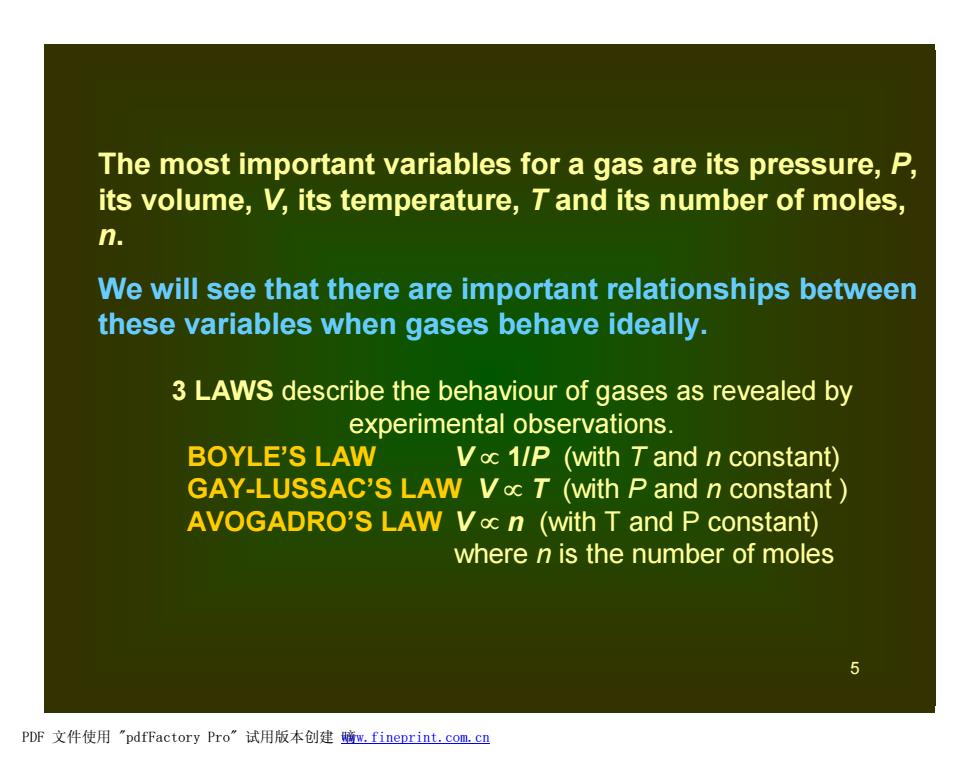
The most important variables for a gas are its pressure,P, its volume,V,its temperature,T and its number of moles, n. We will see that there are important relationships between these variables when gases behave ideally. 3 LAWS describe the behaviour of gases as revealed by experimental observations. BOYLE'S LAW Voc 1/P (with Tand n constant) GAY-LUSSAC'S LAW Vac T (with P and n constant AVOGADRO'S LAW Voc n (with T and P constant) where n is the number of moles PDF文件使用"pdfFactory Pro”试用版本创建暗m,fineprint.com,c里
5 The most important variables for a gas are its pressure, P, its volume, V, its temperature, T and its number of moles, n. We will see that there are important relationships between these variables when gases behave ideally. 3 LAWS describe the behaviour of gases as revealed by experimental observations. BOYLE’S LAW V µ 1/P (with T and n constant) GAY-LUSSAC’S LAW V µ T (with P and n constant ) AVOGADRO’S LAW V µ n (with T and P constant) where n is the number of moles PDF 文件使用 "pdfFactory Pro" 试用版本创建 嘀www.fineprint.com.cn
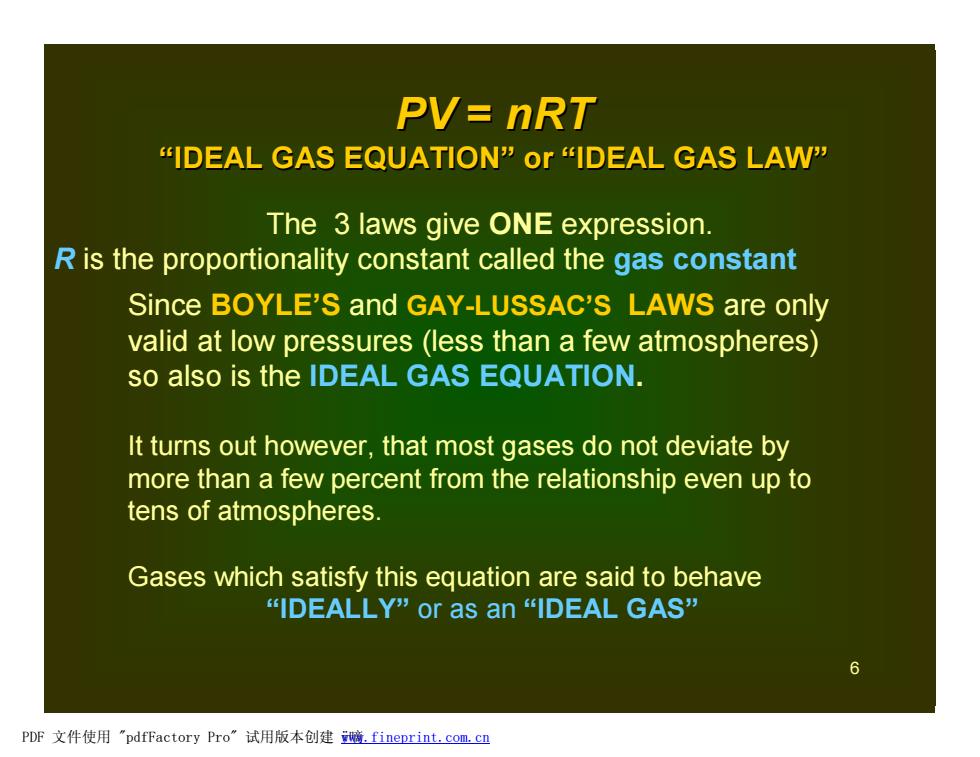
PV=nRT "IDEAL GAS EQUATION"or "IDEAL GAS LAW" The 3 laws give ONE expression. R is the proportionality constant called the gas constant Since BOYLE'S and GAY-LUSSAC'S LAWS are only valid at low pressures (less than a few atmospheres) so also is the IDEAL GAS EQUATION. It turns out however,that most gases do not deviate by more than a few percent from the relationship even up to tens of atmospheres. Gases which satisfy this equation are said to behave “IDEALLY"or as an“IDEAL GAS” PDF文件使用"pdfFactory Pro”试用版本创建脑.fineprint.com,cn
6 The 3 laws give ONE expression. R is the proportionality constant called the gas constant PV = nRT “IDEAL GAS EQUATION” or “IDEAL GAS LAW” Since BOYLE’S and GAY-LUSSAC’S LAWS are only valid at low pressures (less than a few atmospheres) so also is the IDEAL GAS EQUATION. It turns out however, that most gases do not deviate by more than a few percent from the relationship even up to tens of atmospheres. Gases which satisfy this equation are said to behave “IDEALLY” or as an “IDEAL GAS” PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿ嘀www.fineprint.com.cn