
Atomic Absorption Spectrometry (Chapter 9) AAS intrinsically more sensitive than AES similar atomization techniques to AES addition of radiation source high temperature for atomization necessary flame and electrothermal atomization very high temperature for excitation not necessary generally no plasma/arc/spark AAS CEM 333 page 9.1
Atomic Absorption Spectrometry (Chapter 9) • AAS intrinsically more sensitive than AES • similar atomization techniques to AES • addition of radiation source • high temperature for atomization necessary flame and electrothermal atomization • very high temperature for excitation not necessary generally no plasma/arc/spark AAS CEM 333 page 9.1
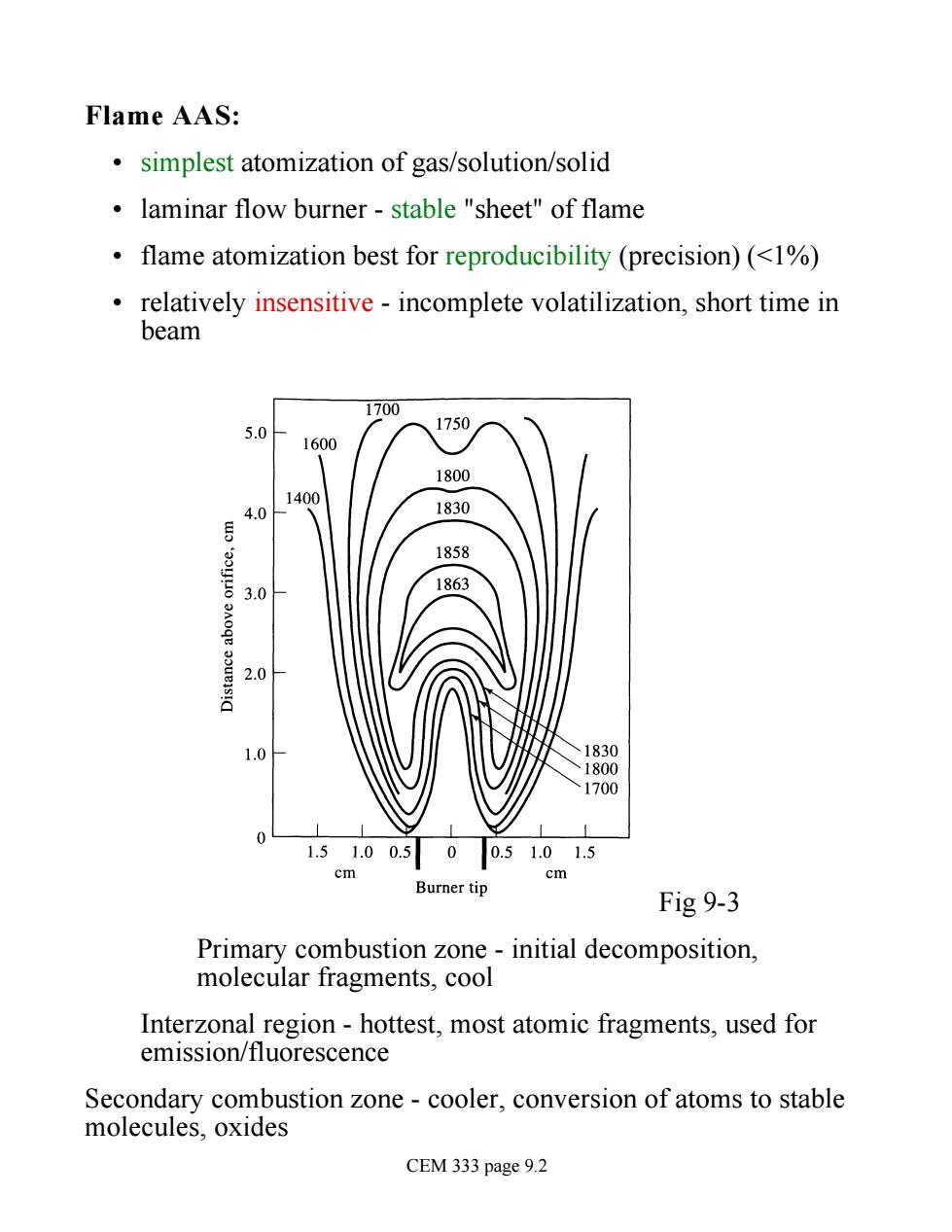
Flame AAS: simplest atomization of gas/solution/solid laminar flow burner-stable "sheet"of flame flame atomization best for reproducibility (precision)(<%) relatively insensitive-incomplete volatilization,short time in beam 700 5.0 1750 1600 1800 40 1830 1858 3.0 2.0 830 70 1.51.00.500.51.01.5 cm Burner tip Fig 9-3 Primary combustion zone-initial decomposition, molecular fragments,cool Interzonal region -hottest,most atomic fragments,used for emission/fluorescence Secondary combustion zone-cooler,conversion of atoms to stable molecules,oxides CEM 333 page 9.2
Flame AAS: • simplest atomization of gas/solution/solid • laminar flow burner - stable "sheet" of flame • flame atomization best for reproducibility (precision) (<1%) • relatively insensitive - incomplete volatilization, short time in beam Fig 9-3 Primary combustion zone - initial decomposition, molecular fragments, cool Interzonal region - hottest, most atomic fragments, used for emission/fluorescence Secondary combustion zone - cooler, conversion of atoms to stable molecules, oxides CEM 333 page 9.2
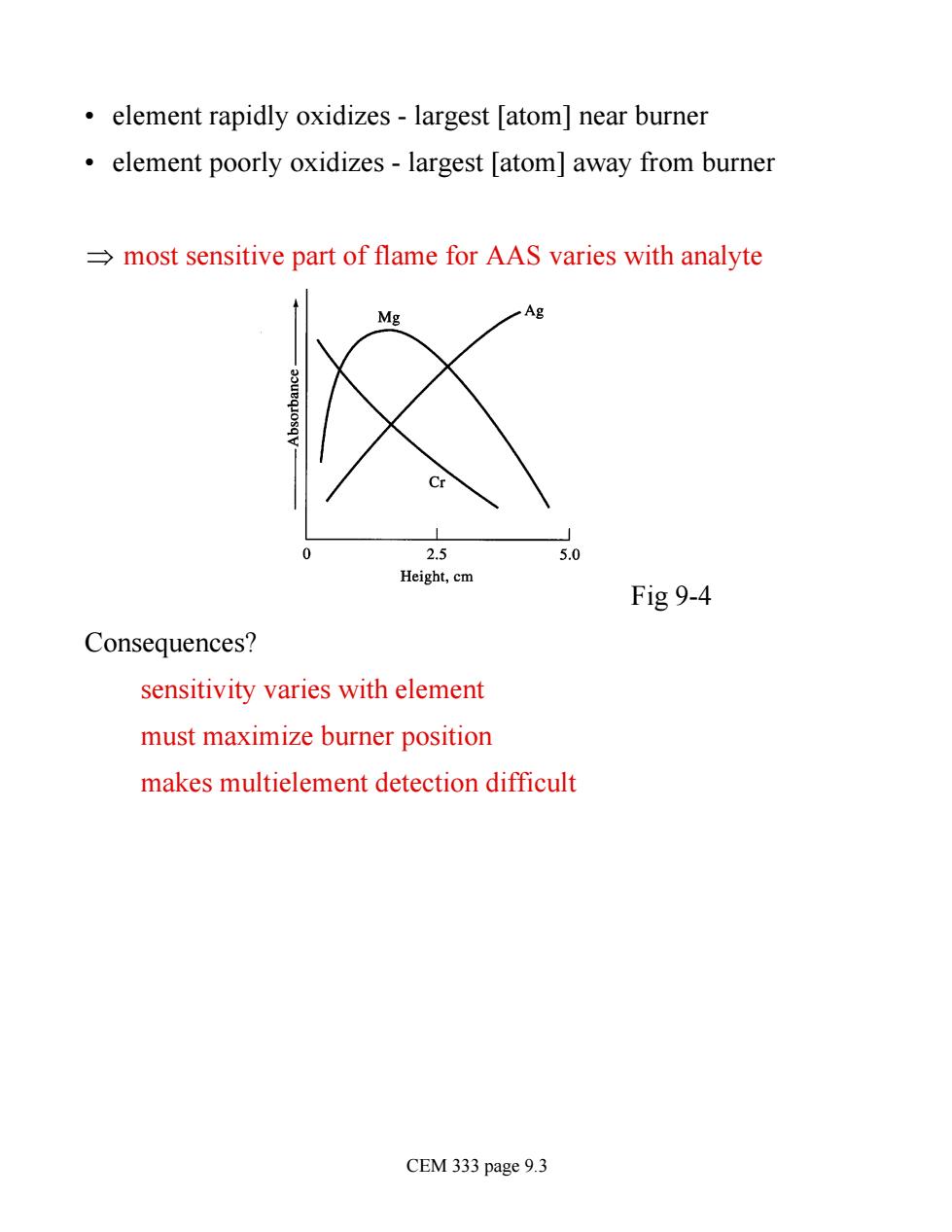
element rapidly oxidizes-largest [atom]near burner element poorly oxidizes-largest [atom]away from burner most sensitive part of flame for AAS varies with analyte 2.5 5.0 Height,cm Fig 9-4 Consequences? sensitivity varies with element must maximize burner position makes multielement detection difficult CEM 333 page 9.3
• element rapidly oxidizes - largest [atom] near burner • element poorly oxidizes - largest [atom] away from burner Þ most sensitive part of flame for AAS varies with analyte Fig 9-4 Consequences? sensitivity varies with element must maximize burner position makes multielement detection difficult CEM 333 page 9.3
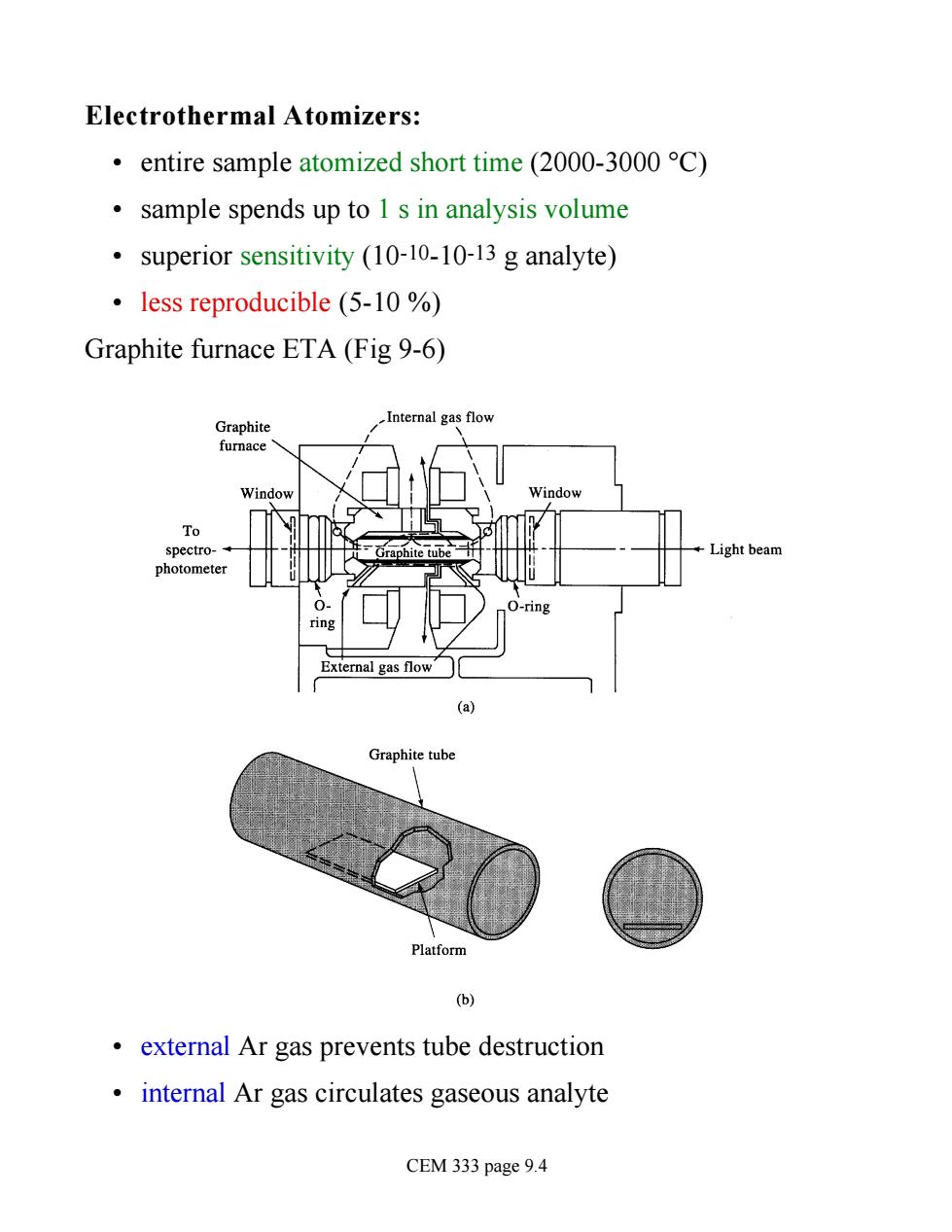
Electrothermal Atomizers: entire sample atomized short time(2000-3000 C) sample spends up to 1 s in analysis volume .superior sensitivity (10-10-10-13g analyte) less reproducible (5-10 % Graphite furnace ETA(Fig 9-6) Graphite Internal gas flow furnace indow To Light beam ring External gas flow☐ Graphite tube Platform 6 external Ar gas prevents tube destruction internal Ar gas circulates gaseous analyte CEM 333 page 9.4
Electrothermal Atomizers: • entire sample atomized short time (2000-3000 °C) • sample spends up to 1 s in analysis volume • superior sensitivity (10-10-10-13 g analyte) • less reproducible (5-10 %) Graphite furnace ETA (Fig 9-6) • external Ar gas prevents tube destruction • internal Ar gas circulates gaseous analyte CEM 333 page 9.4

Three step sample preparation for graphite furnace: (1)Dry evaporation of solvents (10->100 s) (2)Ash-removal of volatile hydroxides,sulfates, carbonates (10-100 s) (3)Fire/Atomize-atomization of remaining analyte(1 s) 0.8 0.7 0.6- 0.5 0.4 Standards(μgmL) 0.2 0.3 03 0.1 0.1 0.05 0.0 Standards Sample Fig 9-7 CEM 333 page 9.5
Three step sample preparation for graphite furnace: (1) Dry - evaporation of solvents (10->100 s) (2) Ash - removal of volatile hydroxides, sulfates, carbonates (10-100 s) (3) Fire/Atomize - atomization of remaining analyte (1 s) Fig 9-7 CEM 333 page 9.5