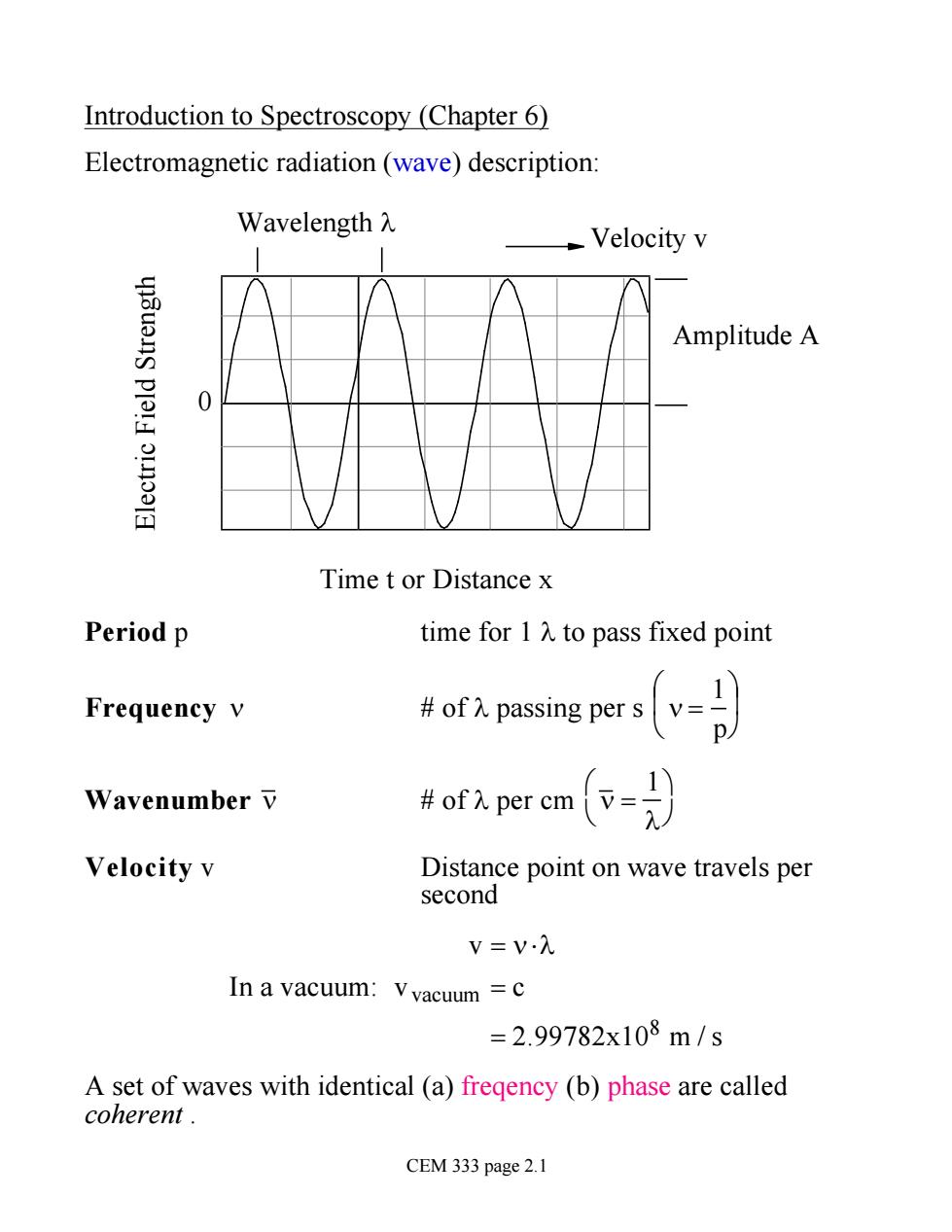
Introduction to Spectroscopy (Chapter 6) Electromagnetic radiation(wave)description: Wavelength入 Velocity v Amplitude A Time t or Distance x Period p time for 1 A to pass fixed point 1 Frequency v of passing per sv= p 1 Wavenumber v #of入per cm Velocity v Distance point on wave travels per second V=V入 In a vacuum:Vvacuum=c =2.99782x108m/s A set of waves with identical (a)fregency (b)phase are called coherent. CEM 333 page 2.1
Introduction to Spectroscopy (Chapter 6) Electromagnetic radiation (wave) description: Electric Field Strength Time t or Distance x Amplitude A Velocity v Wavelength l 0 Period p time for 1 l to pass fixed point Frequency n # of l passing per s n = 1 p æ è ç ö ø ÷ Wavenumber n # of l per cm n = 1 l æ è ö ø Velocity v Distance point on wave travels per second In a vacuum: v = n ×l vvacuum = c = 2.99782x108 m / s A set of waves with identical (a) freqency (b) phase are called coherent . CEM 333 page 2.1

Frequency is always fixed but velocity can vary! Waves slow down in medium(gas,liquid,solid)so v<c V=Vλ Implies A decreases in medium Refractive index1.00 Equation for Wave E=Asin(ot+Φ)where o=2πv electric field frequency amplitude phase angular frequency time Wave description explains certain EM radiation phenomena: transmission reflection and refraction diffraction interference scattering polarization CEM 333 page 2.2
Frequency is always fixed but velocity can vary! Waves slow down in medium (gas, liquid, solid) so v<c v = n ×l Implies l decreases in medium Refractive index h = c v ³ 1.00 Equation for Wave E = Asin(wt + f) where w = 2pn electric field frequency amplitude phase angular frequency time Wave description explains certain EM radiation phenomena: transmission reflection and refraction diffraction interference scattering polarization CEM 333 page 2.2
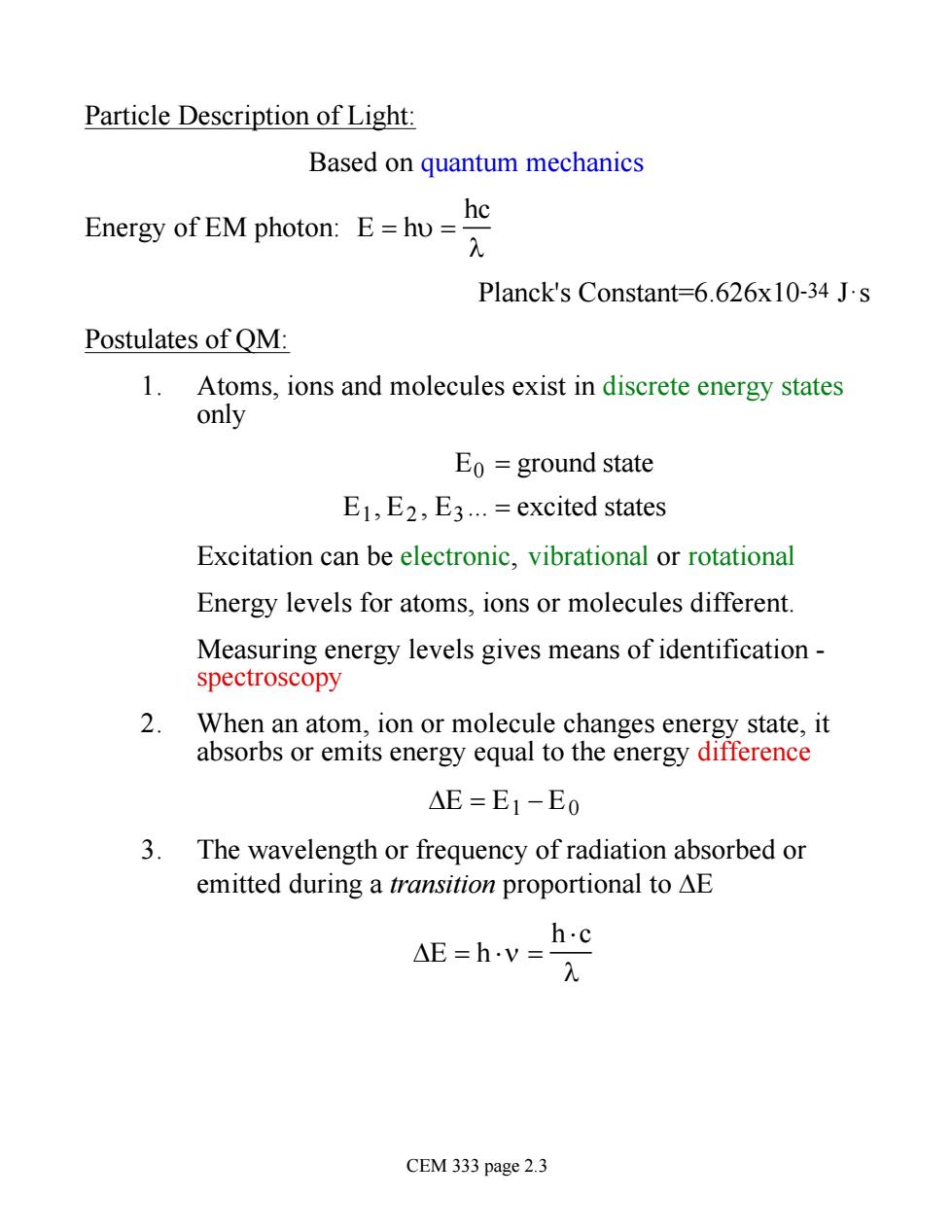
Particle Description of Light: Based on quantum mechanics Energy of EM photon:E=huhe Planck's Constant=6.626x10-34 J.s Postulates of QM 1.Atoms,ions and molecules exist in discrete energy states only Eo=ground state E1,E2,E3.excited states Excitation can be electronic,vibrational or rotational Energy levels for atoms,ions or molecules different. Measuring energy levels gives means of identification- spectroscopy When an atom,ion or molecule changes energy state,it absorbs or emits energy equal to the energy difference AE=E1-Eo 3.The wavelength or frequency of radiation absorbed or emitted during a transition proportional to AE h.c AE=h.v= CEM 333 page 2.3
Particle Description of Light: Based on quantum mechanics Energy of EM photon: E = hu = hc l Planck's Constant=6.626x10-34 J·s Postulates of QM: 1. Atoms, ions and molecules exist in discrete energy states only E0 = ground state E1 , E2 , E3 . = excited states Excitation can be electronic, vibrational or rotational Energy levels for atoms, ions or molecules different. Measuring energy levels gives means of identification - spectroscopy 2. When an atom, ion or molecule changes energy state, it absorbs or emits energy equal to the energy difference DE = E1 - E0 3. The wavelength or frequency of radiation absorbed or emitted during a transition proportional to DE DE = h ×n = h ×c l CEM 333 page 2.3
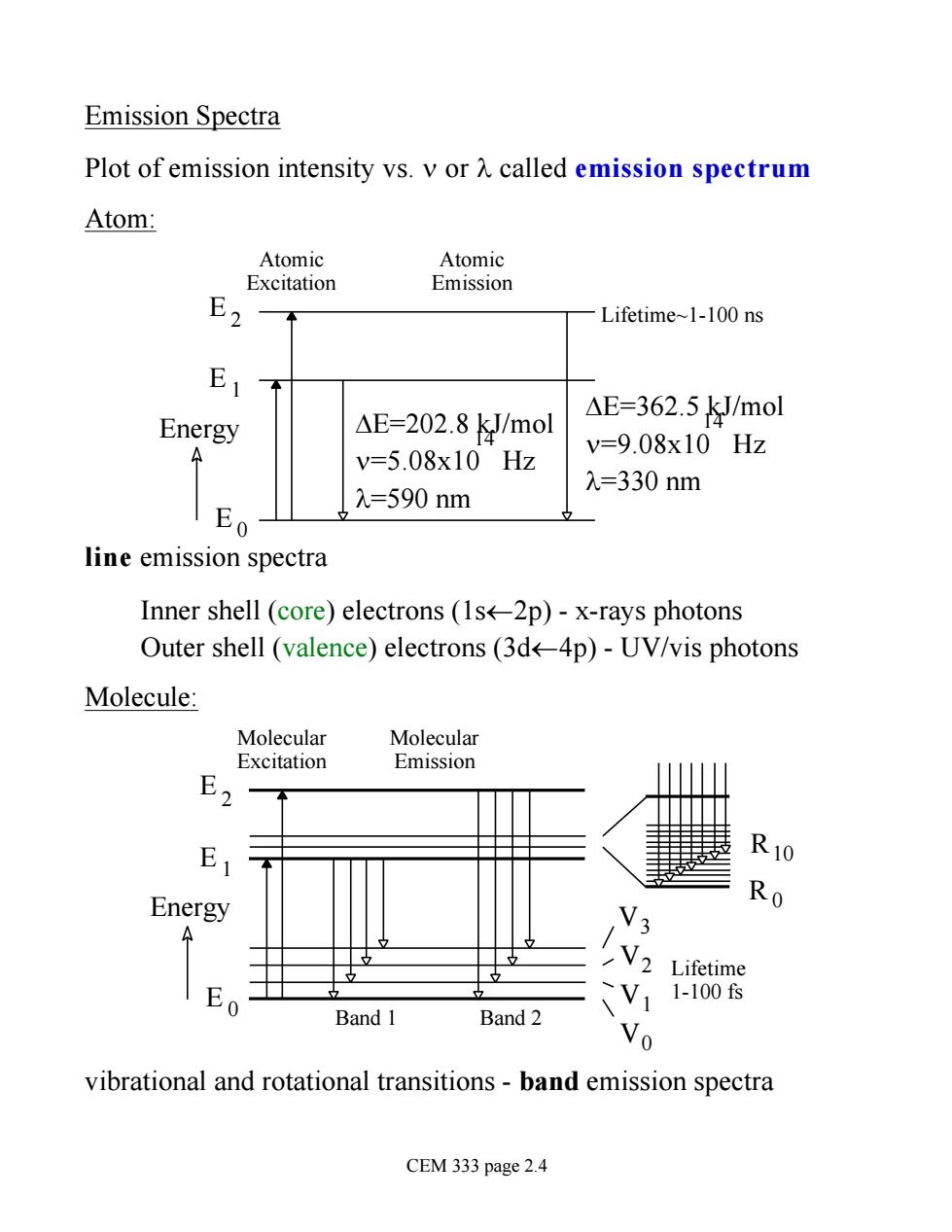
Emission Spectra Plot of emission intensity vs.v or A called emission spectrum Atom: Atomic Atomic Excitation Emission Lifetime~1-100 ns △E=362.5kJ/mol Energy △E=202.8kJ/mol v=9.08x10Hz v=5.08x10Hz \E0 =590nm 入=330nm line emission spectra Inner shell (core)electrons (1s<-2p)-x-rays photons Outer shell (valence)electrons(3d-4p)-UV/vis photons Molecule: Molecular Molecular Excitation 七m1ss1on R10 Energy Ro V3 V2 Lifetime V11-1006 Band 1 Band 2 vibrational and rotational transitions-band emission spectra CEM 333 page 2.4
Emission Spectra Plot of emission intensity vs. n or l called emission spectrum Atom: E 0 E 1 E 2 DE=202.8 kJ/mol n=5.08x10 Hz l=590 nm 14 DE=362.5 kJ/mol n=9.08x10 Hz l=330 nm 14 Energy Atomic Excitation Atomic Emission Lifetime~1-100 ns line emission spectra Inner shell (core) electrons (1s¬2p) - x-rays photons Outer shell (valence) electrons (3d¬4p) - UV/vis photons Molecule: E 0 E 1 E 2 Energy Molecular Excitation Molecular Emission V0 V1 V2 V3 R0 R10 Band 1 Band 2 Lifetime 1-100 fs vibrational and rotational transitions - band emission spectra CEM 333 page 2.4
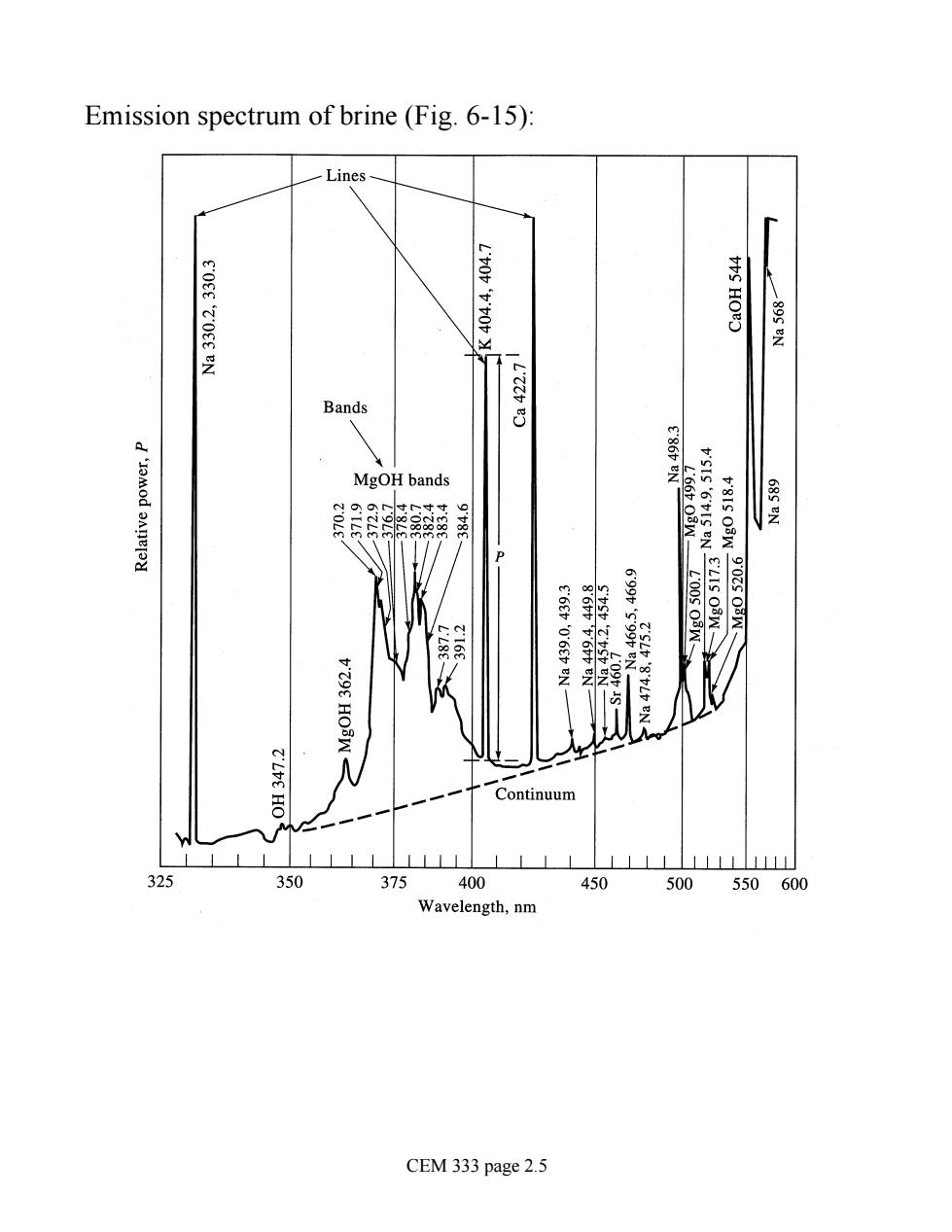
Emission spectrum of brine(Fig.6-15): Bands 22588 MgOH bands 0050- E'LIS O3W- 02s0aN- Continuum 325 350 325 400 450 500 550600 Wavelength,nm CEM 333 page 2.5
Emission spectrum of brine (Fig. 6-15): CEM 333 page 2.5