
Luminescence Spectroscopy (Chapter 15) fluorescence,phosphorescence,chemiluminescence all follow electronic excitation Excited Electronic States: Each electron has unique set of quantum numbers (Pauli Exclusion Principle) n principal (1s,3p. 1 angular momentum (1=0=s,1=1=p.) s spin m magnetic Any two electrons in same orbital(n,1,m)must have different spins S=Zsil Multiplicity:2S+1 (either 1,2,3.) CEM 333 page 5.1
Luminescence Spectroscopy (Chapter 15) fluorescence, phosphorescence, chemiluminescence all follow electronic excitation Excited Electronic States: Each electron has unique set of quantum numbers (Pauli Exclusion Principle) n principal (1s, 3p. ) l angular momentum (l=0=s, l=1=p. ) s spin m magnetic Any two electrons in same orbital (n, l, m) must have different spins s = + 1 2 or - 1 2 S = s å i Multiplicity: 2S+1 (either 1, 2, 3. ) CEM 333 page 5.1
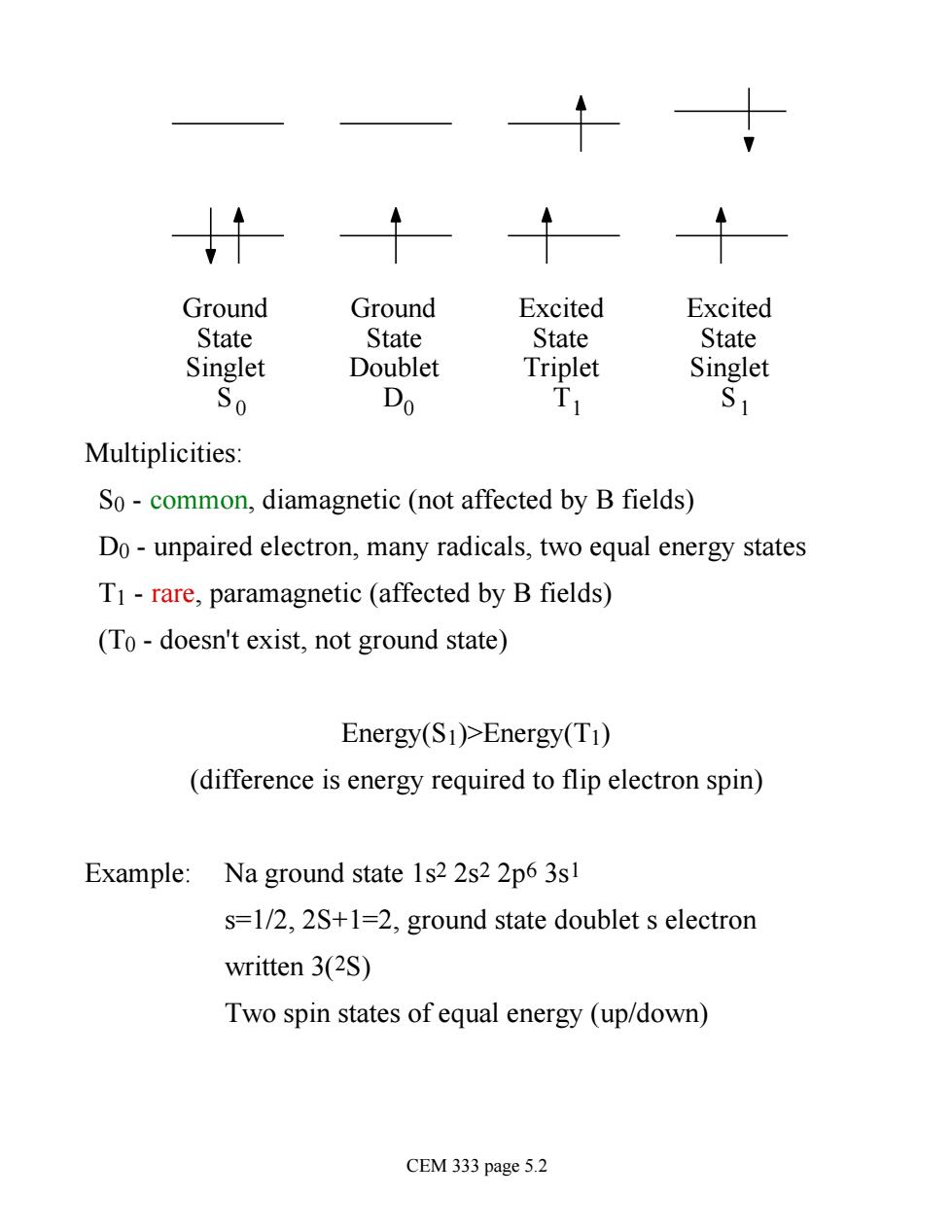
Ground Ground Excited Excited State State State State Singlet Doublet Triplet Singlet So Do Multiplicities: So-common,diamagnetic(not affected by B fields) Do-unpaired electron,many radicals,two equal energy states T1-rare,paramagnetic (affected by B fields) (To-doesn't exist,not ground state) Energy(S1)>Energy(T1) (difference is energy required to flip electron spin) Example:Na ground state 1s2 2s2 2p6 3s s=1/2,2S+1=2,ground state doublet s electron written 3(2S) Two spin states of equal energy (up/down) CEM 333 page 5.2
Ground State Singlet S Ground State Doublet D Excited State Singlet S Excited State Triplet T 0 0 1 1 Multiplicities: S0 - common, diamagnetic (not affected by B fields) D0 - unpaired electron, many radicals, two equal energy states T1 - rare, paramagnetic (affected by B fields) (T0 - doesn't exist, not ground state) Energy(S1)>Energy(T1) (difference is energy required to flip electron spin) Example: Na ground state 1s2 2s2 2p6 3s1 s=1/2, 2S+1=2, ground state doublet s electron written 3(2S) Two spin states of equal energy (up/down) CEM 333 page 5.2

Na Ist excited state 1s2 2s2 2p6 3pl D1 written 3(2P) BUT two spins states? J(total ang.mom)=L+S or L-S now1s22s22p63s1=3(2P12)and3(2P32) Term Symbol 2S+ILJ Na 3p->3s fluorescence two lines at 5896 nm (2P3/2)and 589.0 nm (2P1/2) (SEmision0S1←Absorption_S0)】 CEM 333 page 5.3
Na 1st excited state 1s2 2s2 2p6 3p1 D1 written 3(2P) BUT two spins states? J (total ang. mom)=L+S or L-S now 1s2 2s2 2p6 3s1 = 3(2P1/2) and 3(2P3/2) Term Symbol 2S+1LJ Na 3p®3s fluorescence two lines at 589.6 nm (2P3/2) and 589.0 nm (2P1/2) S1 Emission ¾ ¾ ¾ ¾ ¾ ® S0 S1 Absorption ( ¬ ¾ ¾ ¾ ¾ ¾ S0 ) CEM 333 page 5.3
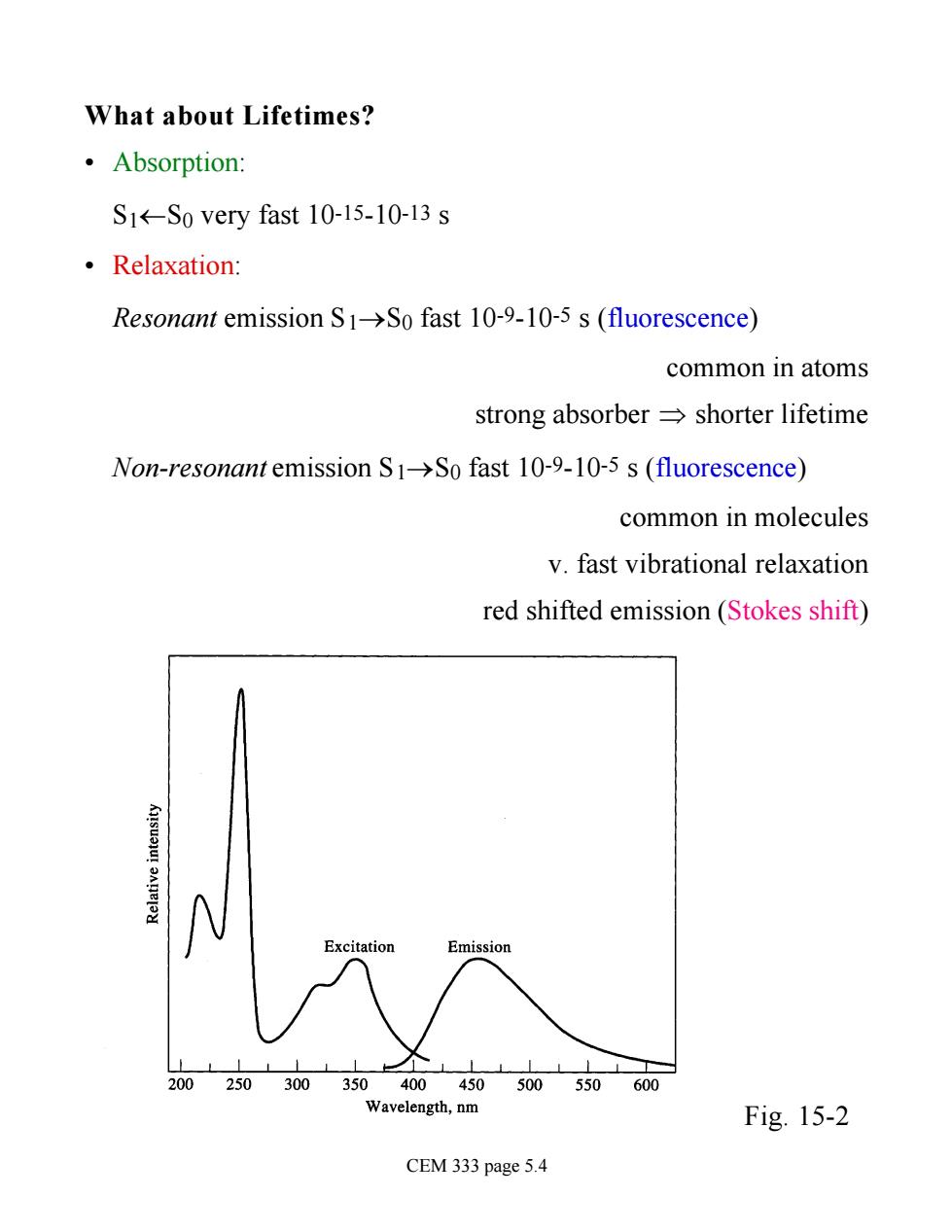
What about Lifetimes? ·Absorption: S1-So very fast 10-15-10-13 s ·Relaxation: Resonant emission S1>So fast 10-9-10-5 s(fluorescence) common in atoms strong absorber=shorter lifetime Non-resonant emission S1->So fast 10-9-10-5 s (fluorescence) common in molecules v.fast vibrational relaxation red shifted emission(Stokes shift) 200250300 Wavelength,nm Fig.15-2 CEM 333 page 5.4
What about Lifetimes? • Absorption: S1¬S0 very fast 10-15-10-13 s • Relaxation: Resonant emission S1®S0 fast 10-9-10-5 s (fluorescence) common in atoms strong absorber Þ shorter lifetime Non-resonant emission S1®S0 fast 10-9-10-5 s (fluorescence) common in molecules v. fast vibrational relaxation red shifted emission (Stokes shift) Fig. 15-2 CEM 333 page 5.4
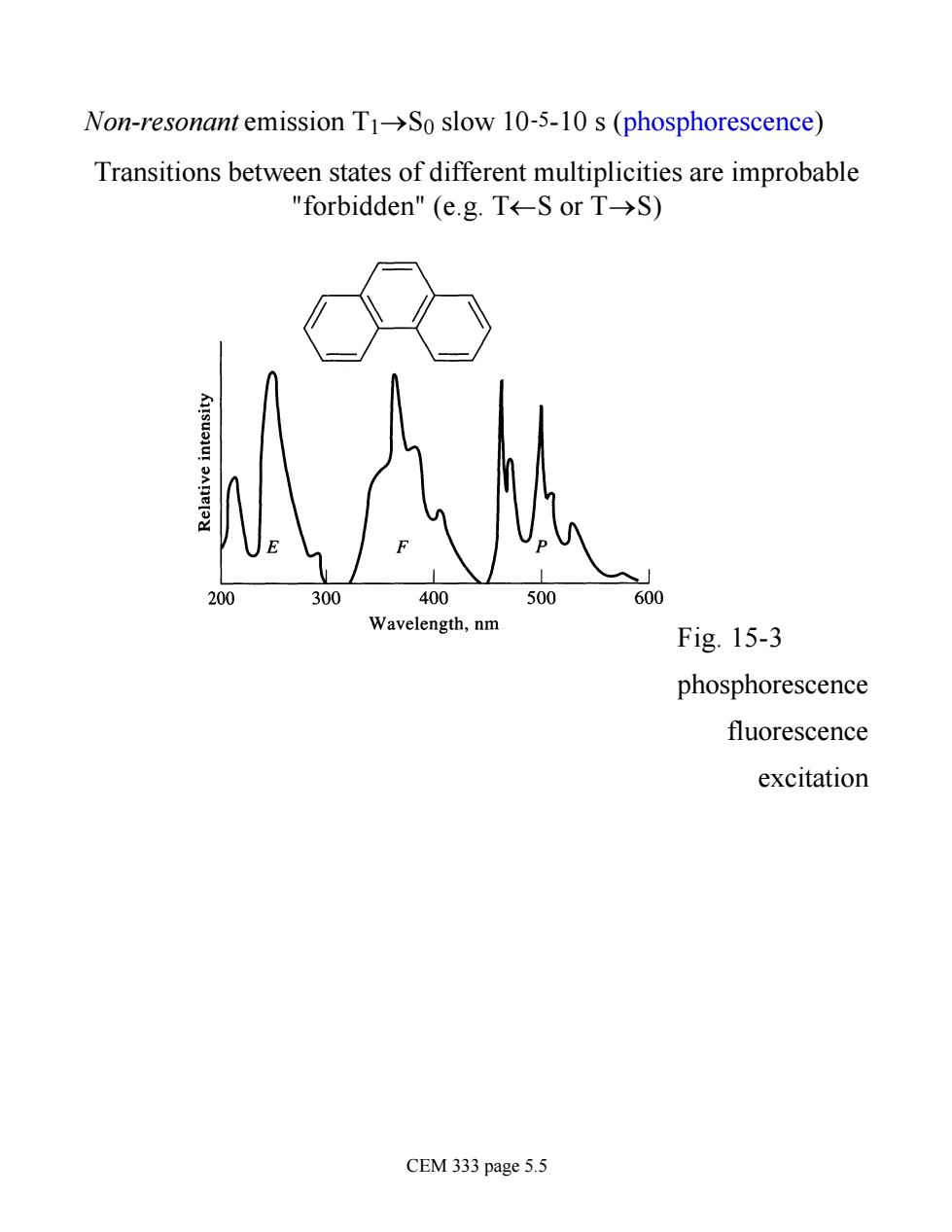
Non-resonant emission T1->So slow 10-5-10 s(phosphorescence) Transitions between states of different multiplicities are improbable "forbidden"(e.g.T←-SorT→S) 300 400 500 600 Wavelength,nm Fig.15-3 phosphorescence fluorescence excitation CEM 333 page 5.5
Non-resonant emission T1®S0 slow 10-5-10 s (phosphorescence) Transitions between states of different multiplicities are improbable "forbidden" (e.g. T¬S or T®S) Fig. 15-3 phosphorescence fluorescence excitation CEM 333 page 5.5