
RC-0 R Naming Aldehydes IUPAC:Replace -e with -al. The aldehyde carbon is number 1. If-CHO is attached to a ring,use the suffix -carbaldehyde. => Chapter 18 6
Chapter 18 6 Naming Aldehydes • IUPAC: Replace -e with -al. • The aldehyde carbon is number 1. • If -CHO is attached to a ring, use the suffix -carbaldehyde. =>
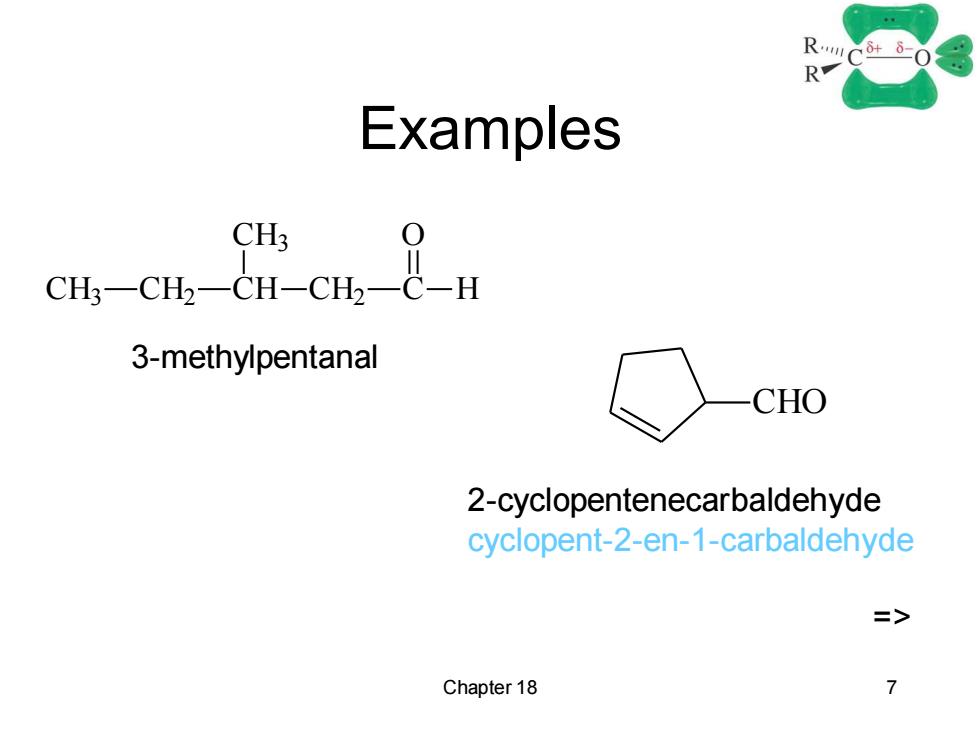
R- Examples CH3 3-methylpentanal CHO 2-cyclopentenecarbaldehyde cyclopent-2-en-1-carbaldehyde => Chapter 18 7
Chapter 18 7 Examples CH3 CH2 CH CH3 CH2 C H O CHO 3-methylpentanal 2-cyclopentenecarbaldehyde cyclopent-2-en-1-carbaldehyde =>
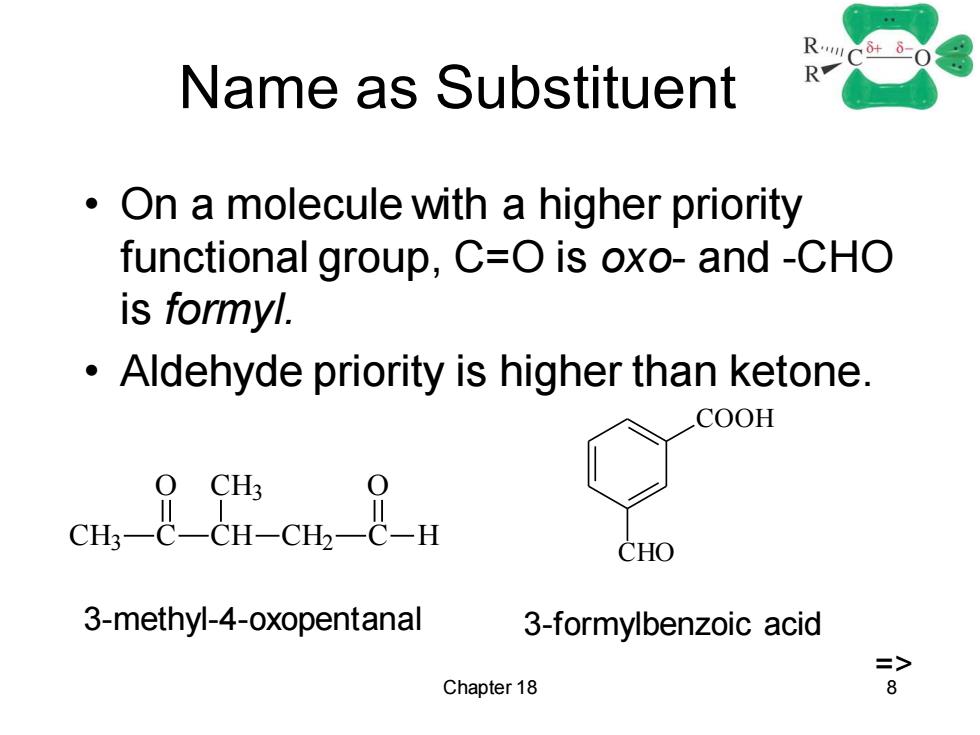
RC+-0 Name as Substituent R On a molecule with a higher priority functional group,C=O is oxo-and -CHO is formyl. Aldehyde priority is higher than ketone. COOH CHO 3-methyl-4-oxopentanal 3-formylbenzoic acid => Chapter 18 8
Chapter 18 8 Name as Substituent • On a molecule with a higher priority functional group, C=O is oxo- and -CHO is formyl. • Aldehyde priority is higher than ketone. CH3 C CH CH3 CH2 C H O O COOH CHO 3-methyl-4-oxopentanal 3-formylbenzoic acid =>
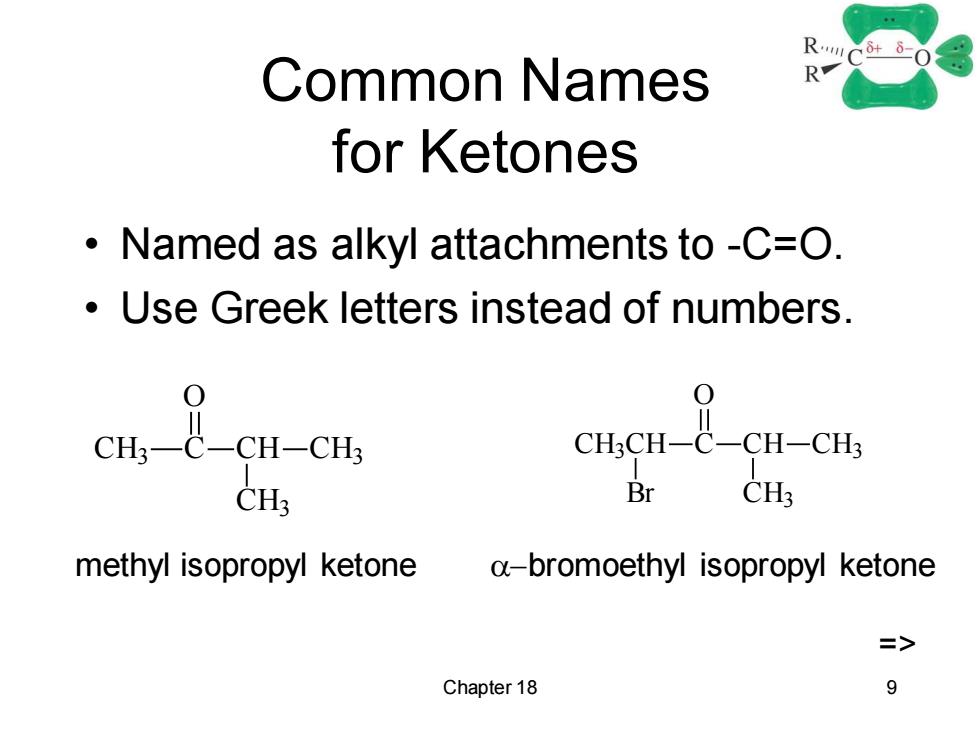
Common Names R for Ketones Named as alkyl attachments to -C=O. Use Greek letters instead of numbers. 0 em t cu-cu CHCH-C-CH-CHs CH3 Br CH3 methyl isopropyl ketone a-bromoethyl isopropyl ketone Chapter 18 9
Chapter 18 9 Common Names for Ketones • Named as alkyl attachments to -C=O. • Use Greek letters instead of numbers. CH3 C O CH CH3 CH3 CH3CH C O CH CH3 CH3 Br methyl isopropyl ketone a-bromoethyl isopropyl ketone =>
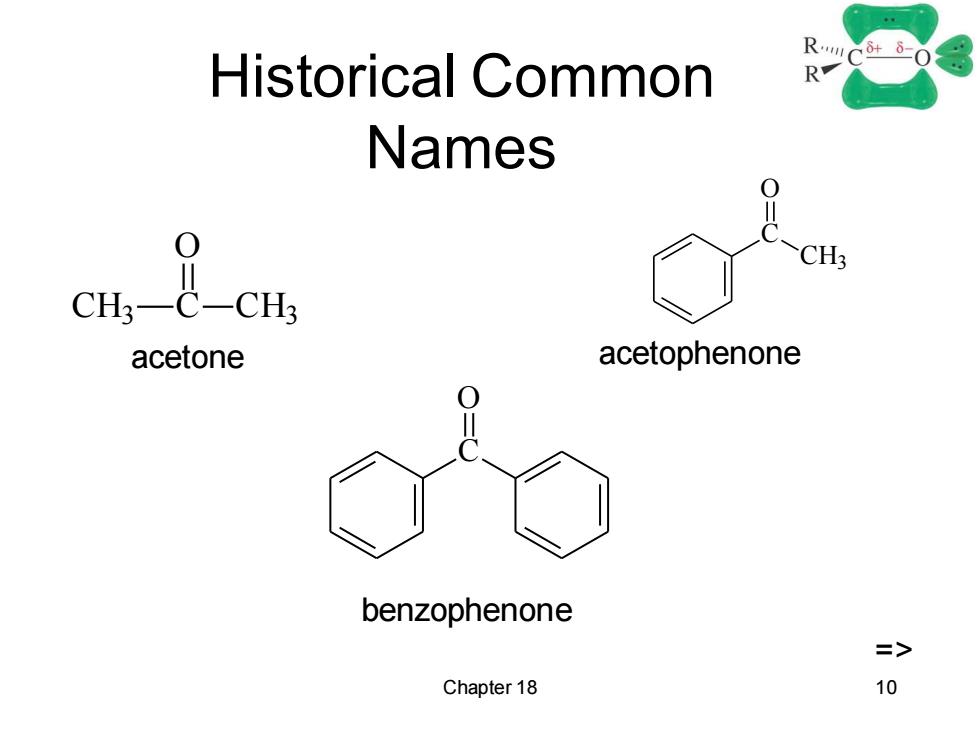
Historical Common RC-0 Names 0 >CH; acetone acetophenone benzophenone => Chapter 18 10
Chapter 18 10 Historical Common Names CH3 C O CH3 C CH3 O C O acetone acetophenone benzophenone =>